
US Keytruda approval scoops one ESMO late-breaker

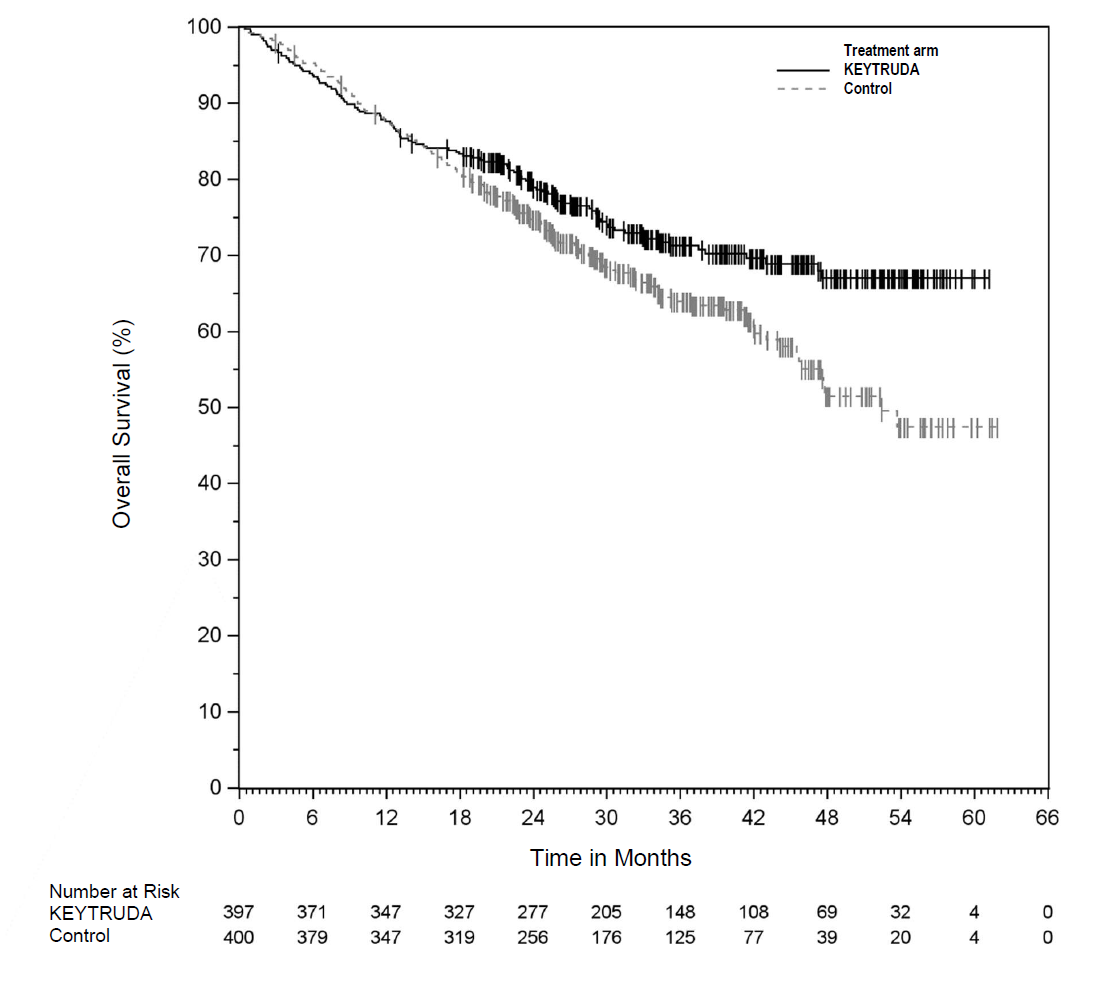
After Keytruda’s periadjuvant lung cancer study Keynote-671 hit its co-primary overall survival endpoint last week Merck & Co promised full data presentation at an ESMO late-breaker, but this has been scooped by yesterday’s US approval. According to just updated US prescribing information, in Keynote-671 neoadjuvant Keytruda plus chemo followed by adjuvant Keytruda monotherapy in stage II to IIIB NSCLC reduced risk of death by 28% versus placebo, with a p value of 0.0103. Data on the trial’s earlier hit on event-free survival had already been published, as have EFS data from AstraZeneca’s rival Aegean trial of Imfinzi, though the latter study doesn’t yet appear to have been submitted to regulators, and is continuing to its OS assessment. In neoadjuvant NSCLC Keytruda will now compete against Bristol Myers Squibb’s Opdivo, which has a US label backed by the chemo-combo Checkmate-816 trial; that didn’t have an adjuvant stage, however, and comprised stage IB-IIIA patients. The ESMO presentation on Keynote-671 takes place this Friday under abstract LBA56.
Overall survival curves in Keynote-671
1230













