ASCO 2023 – Behind Astellas comes Elevation
Elevation’s anti-Claudin18.2 antibody-drug conjugate generates some justified optimism, but Zai Lab’s zolbetuximab challenger is out of luck.
Elevation’s anti-Claudin18.2 antibody-drug conjugate generates some justified optimism, but Zai Lab’s zolbetuximab challenger is out of luck.

Astellas’s zolbetuximab has two pivotal study hits under its belt, and will go before regulators later this year, but behind it the next wave of Claudin18.2-targeting projects is already building. Investor interest has been piqued by Elevation Oncology’s SYSA1801, for instance, and today’s ASCO presentation lent weight to some of this enthusiasm.
The news was not so good for Zai Lab’s ZL-1211, which despite being enhanced for activity showed no responses in its Asco poster today. One question for Elevation, a micro-cap valued at just $86m, is whether SYSA1801 can, in spite of being an antibody-drug conjugate, maintain a manageable safety profile.
The signs so far are that nausea and vomiting are the most common treatment-related adverse events, and one concern is in that two of seven subjects given the highest dose of SYSA1801, 3mg/kg this toxicity was dose limiting.
33 have been enrolled into this trial total, and the Asco presentation contained the same 5 November 2022 data cutoff as detailed in the abstract that had sent Elevation up 67% a week ago. A major question in why Elevation brought to ASCO data that were nearly seven months old – especially as many of its responses remain unconfirmed.
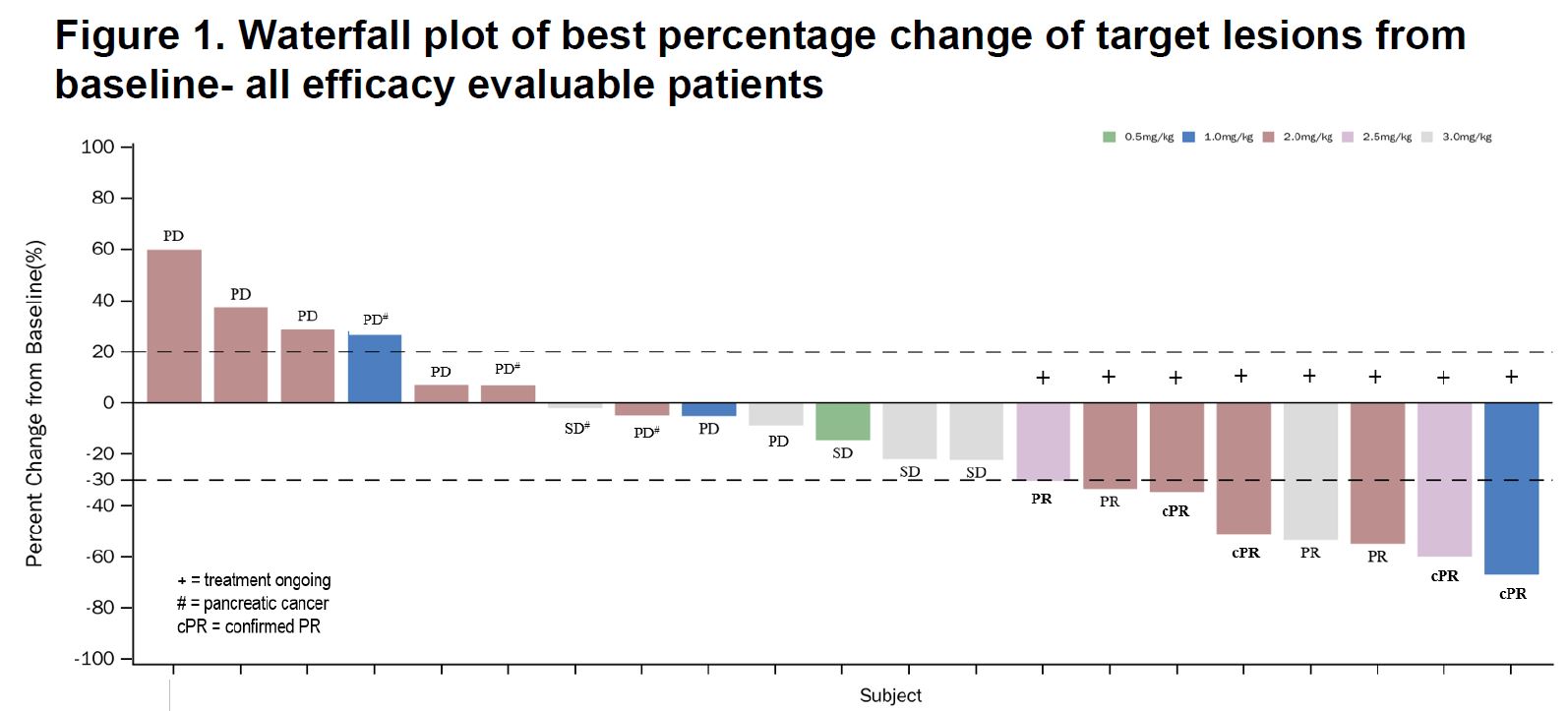
Since the company has an undemanding valuation perhaps some enthusiasm is warranted. There were 18 evaluable Claudin18.2-positive patients at data cutoff, 17 of whom had advanced gastric cancer, and among these there were eight partial responses, four of which were independently confirmed.
An ORR of 47% looks impressive here given that this is a monotherapy study. In comparison zolbetuximab’s pivotal Spotlight trial, in Claudin18.2-positive gastric cancer, gave a 61% ORR, though this was a chemo-combo study, and curiously chemo alone gave 62%; that puzzling finding only slightly detracted from the trial’s demonstrated survival benefit.
Naked antibody
SYSA1801 is an ADC, but Zai’s ZL-1211 and zolbetuximab are both naked MAbs. However, unlike the Astellas project ZL-1211 has been modified with site mutations introduced into its Fc region, with the aim of enhancing antibody-dependent cellular cytotoxicity.
The poster presented at Asco showed 10 evaluable Claudin18.2-positive patients, but the best result seen was stable disease with 25% tumour reduction in a gastric cancer subject. Serious nausea and vomiting occurred in one of 19 safety-evaluable subjects, but in none given high ZL-1211 doses, so perhaps Zai can still manage to dose ZL-1211 higher.
255













