Moderna moves on swiftly with its neoantigen immunotherapy
Lung cancer will soon join melanoma as a phase 3 indication for mRNA-4157, an asset Merck & Co last year endorsed to the tune of $250m.
Lung cancer will soon join melanoma as a phase 3 indication for mRNA-4157, an asset Merck & Co last year endorsed to the tune of $250m.

Having moved the cancer immunotherapy mRNA-4157 into a pivotal melanoma study in June, Moderna has set its sights on non-small cell lung cancer. A phase 3 NSCLC trial in a similar adjuvant setting will begin later this year, the company told yesterday’s R&D day.
The project has moved swiftly through the clinic, having entered phase 3 in melanoma just seven months after yielding its first topline data last December. There is clear clinical backing for mRNA-4157’s pivotal melanoma trial, with April’s AACR presentation largely justifying the earlier enthusiasm, though it is evident that support for the NSCLC indication is a little weaker.
For now such support is limited to results from the phase 1 Keynote-603 basket trial in which all 11 stage IA-IIB NSCLC patients given adjuvant mRNA-4157 monotherapy remained recurrence-free for 25-135 weeks. There is a lot riding on mRNA-4157, a personalised neoantigen immunotherapy that was endorsed last year when Merck & Co opted into its development in a deal worth $250m up front.
Moderna yesterday gave away little about the design of the planned phase 3 NSCLC trial, so it is as yet unclear whether this will involve mRNA-4157 monotherapy or a Keytruda combo, though presumably the comparator will be Keytruda alone. Moderna says the trial will enrol patients with resected stage II-IIIB NSCLC who have received adjuvant chemotherapy and have had no disease recurrence.
Meanwhile, the pivotal trial that begun in July tests mRNA-4157 (also coded V940) plus Keytruda in stage IIB-IV melanoma that is untreated apart from having been resected, and tests recurrence-free survival versus Keytruda alone as its primary endpoint. In the fourth quarter Moderna also promises an update on results from Keynote-942, its phase 2 melanoma trial.
It was Keynote-942 whose AACR presentation appeared to justify Merck’s enthusiasm. The data confirmed an earlier toplined 44% reduction in disease recurrence or death, allayed fears over possible underperformance of the Keytruda monotherapy comparator, and suggested that the study’s main limitation was simply its relatively small size.
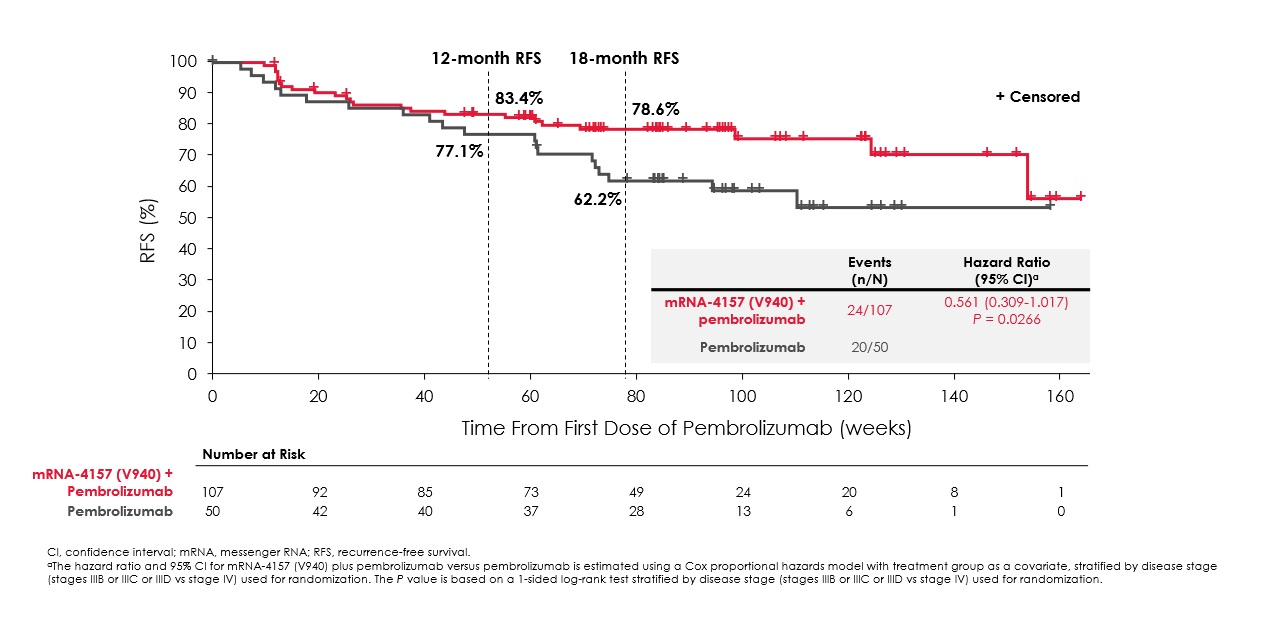
RFS curves in Keynote-942
mRNA-4157 is a personalised mRNA therapeutic created on demand and encoding up to 34 neoantigens present in each patient’s tumour. Keytruda is approved for adjuvant melanoma, both for stage III (based on the result of the Keynote-054 study) and for less advanced stage IIB/C disease (Keynote-716), as well as for stage adjuvant IB-IIIA NSCLC (Keynote-091).
It was Keynote-054, where 18-month RFS was 71%, that had hinted that the 62% result for Keytruda monotherapy in Keynote-942 was an underperformance. However, Keynote-942 included more advanced stage IV melanoma patients, in addition to the stage IIIs to which Keynote-054 was limited, a fact that might have contributed to a lesser benefit overall in Keynote-942.
Bristol Myers Squibb’s Opdivo is approved in adjuvant treatment of stage IV as well as stage III melanoma, on the basis of the Checkmate-238 trial, in which 18-month RFS was around 65%. And, just for good measure, Roche’s Tecentriq is approved for adjuvant stage II-IIIA NSCLC, though only in PD-L1 ≥1% expressers.
Cross-trial comparisons
Adjuvant treatment of stage III-IV melanoma… | |||
|---|---|---|---|
| Study | |||
| Population | Stage IIIB/C & IV | Stage IIIA/B/C | Stage IIIB/C/D & IV |
| 18mth RFS for mRNA-4157 + Keytruda | NA | NA | 79% |
| 18mth RFS for Keytruda | NA | 71% | 62% |
| 18mth RFS for Opdivo | 65% | NA | NA |
| 18mth RFS for chemo | 52% | 53% | NA |
| Statistics vs control for RFS across the entire study | HR=0.65 (p<0.0001) | HR=0.57 (p<0.001) | HR=0.56 (=0.0266)** |
...and of stage I-III NSCLC | |||
|---|---|---|---|
| Study | ? | ||
| Population (after adjuvant chemo) | Stage IB-IIIA | PD-L1≥1% stage II-IIIA | Stage II-IIIB |
| 18mth DFS for mRNA-4157 (+ Keytruda?) | NA | NA | ? |
| 18mth DFS for Keytruda | 73% | NA | ? |
| 18mth DFS for Tecentriq | NA | 80% | NA |
| 18mth DFS for placebo | 64% | 67% | NA |
| Statistics vs control for DFS across the entire study | HR=0.73 | HR=0.66 (p=0.004) | ? |
Notes: *ph2 study, others are ph3; **one-sided p value, and 95% CI upper bound of 1.017. RFS=relapse-free survival. DFS=disease-free survival. Source: product labels & AACR.
mRNA-4157 is noteworthy on account of being the first neoantigen immunotherapeutic of its type to show anything approaching meaningful efficacy, and it is now the first such agent to be studied in phase 3.
At AACR 2020 BioNTech’s Roche-partnered autogene cevumeran (RO7198457) was said to have “modest activity in a heavily pretreated dose-escalation patient population”. The partners have moved it into the phase 2 Imcode-001 study in combination with Keytruda in melanoma, though notably an adjuvant NSCLC trial was withdrawn before enrolling any patients.
Also worth watching here is Gritstone Bio, whose Granite-001 project (GRT-C901 plus GRT-R902 vectors) is in phase 2/3. Gritstone claims that its technology has a strong T-cell priming effect, aiming to make “cold” tumours “hot”, which is why it is testing Granite in colorectal cancer rather than in a relatively immunogenic setting like melanoma.
The first randomised data from the phase 2 part of Gritstone’s study are due in the first quarter of 2024.
1568













