
Akeso’s CD47 heads for phase 3

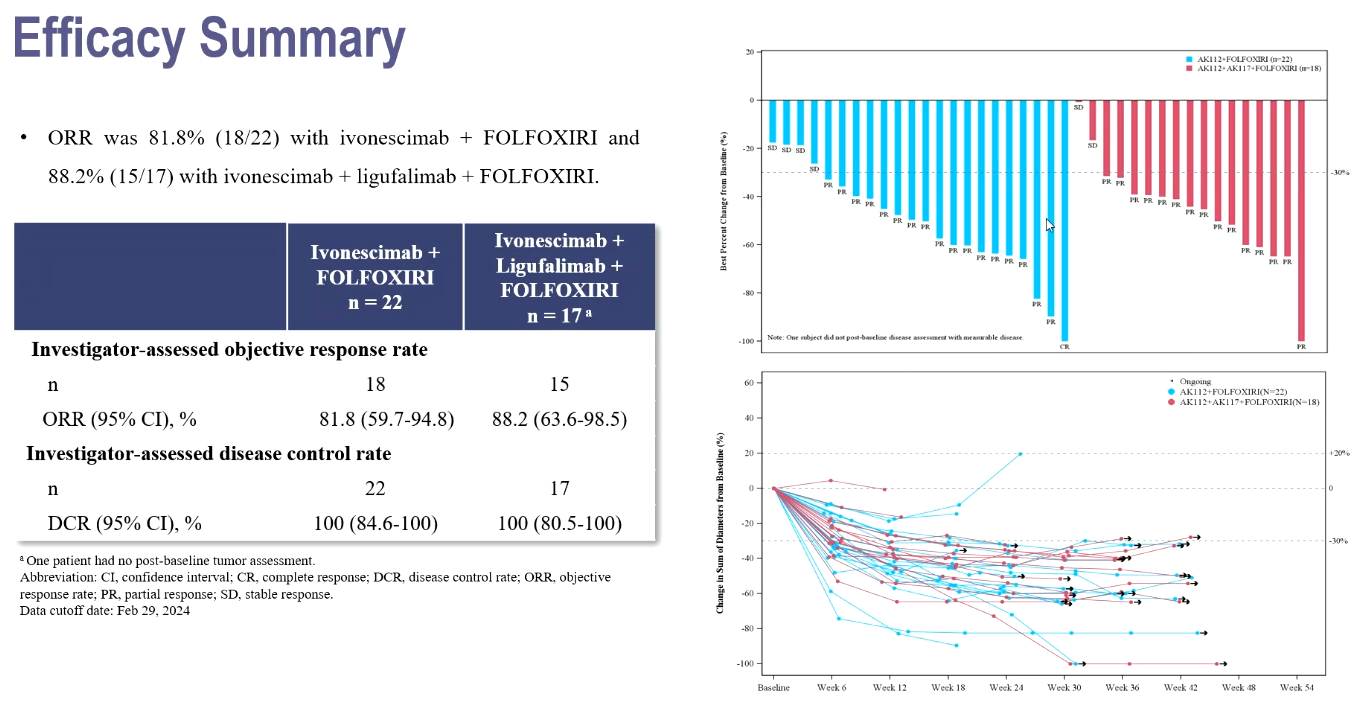
Perhaps emboldened by a success at the recent ESMO conference, Akeso is taking its anti-CD47 MAb ligufalimab into a phase 3 trial, a new listing on the clinicaltrials.gov registry has revealed. However, this will not be in colorectal cancer, the setting of the ESMO success, but rather in first-line, PD-L1-positive head and neck squamous cell carcinoma. In both cases, however, there is a vital element: ligufalimab is being combined with ivonescimab, the high-profile Summit-partnered anti-VEGF x PD-1 MAb that emerged as a key winner of ESMO. The new trial, due to start in China next month, will compare ligufalimab plus ivonescimab versus Keytruda, and test overall survival as sole primary endpoint. Keytruda isn’t approved in China for head and neck cancer, but does carry this label in the US, as a first-line chemo combo, and as monotherapy for first (PD-L1-positive) and second-line (all-comers) use. A benchmark for ligufalimab plus ivonescimab, therefore, is the 12.3 months of median OS that Keytruda scored in PD-L1 ≥1% expressers in the first-line Keynote-048 study. Meanwhile, at ESMO ligufalimab plus ivonescimab and chemo yielded 15 responses in 17 first-line colorectal cancer patients; chemo plus ivonescimab alone scored an ORR of 82%.
Phase 2 data in 1st-line colorectal cancer
1576













