
Toxicity hits BioNTech’s MediLink tie-up
A mysterious ASCO poster, patient deaths and now clinical hold; where next for BioNTech’s ADC deal?
A mysterious ASCO poster, patient deaths and now clinical hold; where next for BioNTech’s ADC deal?

This week’s partial US clinical hold on BNT326, with the FDA flagging concerns about the risk of “illness or serious injury”, throws a spotlight on a phase 1 study presented at ASCO on this anti-HER3 ADC project, which BioNTech licensed from China’s MediLink Therapeutics last October for $70m up front.
The problems with BNT326 are relevant in light of BioNTech’s quest to become a cancer company, of which ADCs are a key part. They might also raise the question of why the German group chose to work on HER3 with MediLink rather than with DualityBio, which is collaborating with BioNTech on several other projects but whose own anti-HER3 ADC is currently unpartnered.
At the time BioNTech licensed BNT326, currently in phase 2, from China’s MediLink in October, Merck & Co made a huge bet on Daiichi Sankyo’s patritumab deruxtecan – now the industry’s most advanced anti-HER3 ADC; Bristol Myers Squibb also got into this mechanism, in December paying $800m up front to license SystImmune’s izalontamab brengitecan, which also hits EGFR.
High-dose deaths
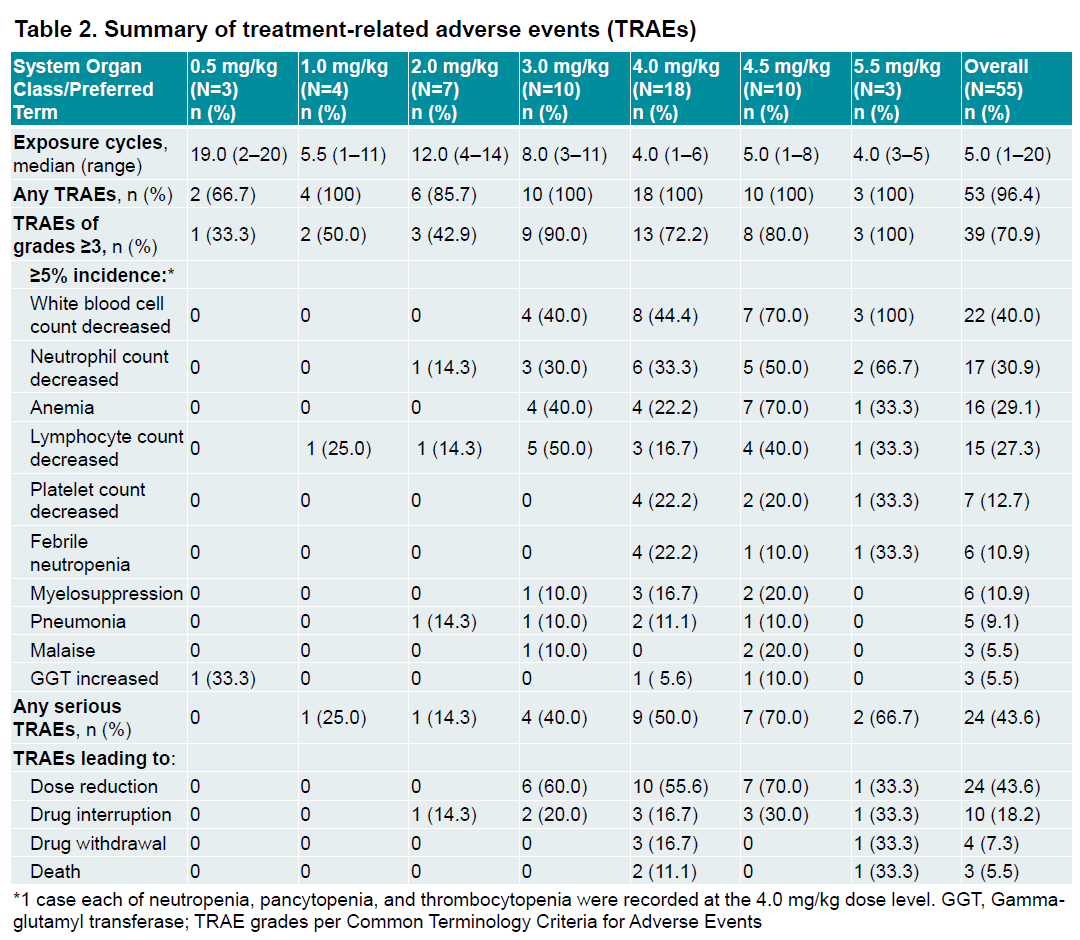
Earlier this month ASCO saw data from BNT326’s first-in-human trial, being carried out in the US and China by MediLink. The study, in patients with late-line EGFR-mutant NSCLC and HR-positive, HER2-negative breast cancer, tested doses ranging from 0.5mg/kg to 5.5mg/kg.
There were three deaths deemed treatment-related, at 4.0mg/kg and 5.5mg/kg. The two fatalities at 4.0mg/kg were due to sepsis after febrile neutropenia and pancytopenia, and the one at 5.5mg/kg was caused by interstitial pneumonia after Covid-19 infection.
The poster on that MediLink-sponsored trial appeared on the ASCO poster board during the meeting, but – unlike other ASCO posters – wasn’t uploaded to the conference website so can no longer be viewed. However, after a request, BioNTech sent the poster to ApexOnco by email. At the time of publication, ASCO hadn't responded to questions about why the poster isn't available online.
The poster shows the most common grade 3 or higher adverse event to be white blood cell count decreases, seen in 100% of patients receiving 5.5mg/kg, in 70% of the 4.5mg/kg group and in 44% of the 4.0mg/kg cohort. These numbers weren’t shown in BioNTech’s ASCO presentation for analysts, which includes only a summary of adverse events.
The German group noted that further development would focus on dose levels below 4.0mg/kg, where it deemed the project’s safety profile “manageable”.
As for efficacy, BioNTech claimed an ORR of 42% among 52 evaluable patients; however, taking into account three additional patients included in the swimmer’s plot, who died or came off trial before being assessed, gives an ORR of 40%. Breaking out the results by disease type gives an ORR of 47% in breast cancer (7 of 15 patients responding) and 38% in NSCLC (15 of 40).
This compares favourably to the 30% ORR seen with Daiichi/Merck’s patritumab deruxtecan, in the Herthena-Lung01 trial in late-line EGFRm NSCLC. That asset is awaiting an FDA approval decision. Meanwhile, Bristol/SysImmune’s iza-can has shown an ORR of 63% in late-line EGFRm NSCLC.
Still, any BNT326 cross-trial superiority won't matter if BioNTech and MediLink can’t find a therapeutic window. BNT326 is also in a phase 2 Chinese solid tumour study, which began recruiting last year. The FDA also cited as a reason for the clinical hold deaths in this trial, known as YL202-CN-201-01, although no results are publicly available.
HER3 hot
A look at the rest of the HER3 ADC pipeline doesn’t throw up an obvious explanation for why BNT326 might have a toxicity problem. Like all of the other clinical-stage projects, this project uses a cleavable linker, and a topoisomerase 1 inhibitor, albeit a novel one.
The MediLink project also utilises what the company calls its tumour microenvironment “activable” linker-payload (Tmalin) technology, which it says could promote accumulation in the tumour and reduce off-target toxicity.
This technology and payload is shared by MediLink’s other ADCs, including the cMet-targeting YL211, which Roche licensed for $50m up front in January; that asset recently went into phase 1.
With ADCs remaining hot, any more information about BNT326’s apparent problems will be eagerly awaited. In the meantime, BioNTech looks to have fallen even further behind in HER3.
Clinical-stage HER3-targeting ADCs
| Project | Company | Linker | Payload | DAR | Status |
|---|---|---|---|---|---|
| Patritumab deruxtecan | Daiichi Sankyo/ Merck & Co | Cleavable, cathepsin | Deruxtecan (topoisomerase 1 inhibitor) | 8 | PDUFA date 26 Jun, based on ph2 Herthena-Lung01 in EGFRm NSCLC |
| Izalontamab brengitecan* | SystImmune/ Bristol Myers Squibb | Cleavable, cathepsin | Ed-04 (topoisomerase 1 inhibitor) | 8 | Various China ph3s |
| SHR-A2009 | Luzsana (Jiangsu HengRui) | Cleavable, protease | Rezetecan (topoisomerase 1 inhibitor) | 4 | Ph1/2 China trial in solid tumours |
| DB-1310 | Duality Biologics | Cleavable, cathepsin | P1021 (topoisomerase 1 inhibitor) | 8 | Ph1/2 US/China trial in solid tumours |
| IBI133 | Innovent Biologics | Cleavable | Exatecan & derivative (topoisomerase 1 inhibitor) | ? | Ph1/2 Aus/China trial in solid tumours |
| BNT326 (YL202) | BioNTech/ MediLink | Cleavable, protease or TME-specific trigger | YL0010014 (topoisomerase 1 inhibitor) | 8 | Ph1 in EGFRm NSCLC or HR+/HER2- breast cancer on hold |
| AMT-562 | Multitude Therapeutics | Cleavable, cathepsin | Exatecan & derivative (topoisomerase 1 inhibitor) | 8 | Ph1 Aus/China study in solid tumours |
| JSKN016** | Alphamab Oncology | Cleavable, cathepsin | Camptothecin analogue (topoisomerase 1 inhibitor) | 3.5 | Ph1 China study |
| SIBP-A13 | Shanghai Institute Of Biological Products | Cleavable | Camptothecin analogue (topoisomerase 1 inhibitor) | ? | Ph1 China study |
Note: *anti-EGFR x HER3 bispecific ADC; **anti-HER3 x TROP2 bispecific ADC. Source: OncologyPipeline.
2876













