Essa terminates an N-terminal journey
After beating "historical" Xtandi masofaniten fails to beat actual Xtandi.
After beating "historical" Xtandi masofaniten fails to beat actual Xtandi.
Barely a month after piquing investor interest at ESMO with dose-escalation data of its lead asset, masofaniten, Essa Pharma has terminated all trials of this project.
That's bad news for the company, whose central mission was to develop prostate cancer therapeutics that hit not the ligand-binding domain of the androgen receptor (AR), but rather its N-terminal region. Masofaniten was the most advanced such N-terminal domain AR inhibitor, and its effective discontinuation saw Essa stock crash 73% on Friday.
Essa claimed that masofaniten could avoid resistance mechanisms that develop in response to drugs that primarily affect AR ligand binding, including Zytiga, which inhibits androgen synthesis, and Xtandi, Erleada and Nubeqa, which block androgen binding.
No benefit
Unfortunately for the company its phase 1/2 study in metastatic castration-resistant prostate cancer patients naive to second-generation antiandrogens, which pitted masofaniten plus Xtandi versus Xtandi alone, has manifestly failed to show such a benefit.
The proportion of patients achieving a PSA90 response (a PSA decline of at least 90%), which Essa described as the phase 2 portion's primary efficacy endpoint, showed no advantage, with Xtandi numerically beating the masofaniten combo by 73% versus 64% across 52 patients with at least one PSA measurement from baseline.
This phase 2 dose-expansion stage focused on masofaniten 600mg twice daily combined with daily Xtandi at 160mg. Other efficacy endpoints, namely PSA50 response, as well as PSA50 response at 90 days and PSA90 at 90 days, also failed to show a benefit for masofaniten, Essa said after market close on Thursday.
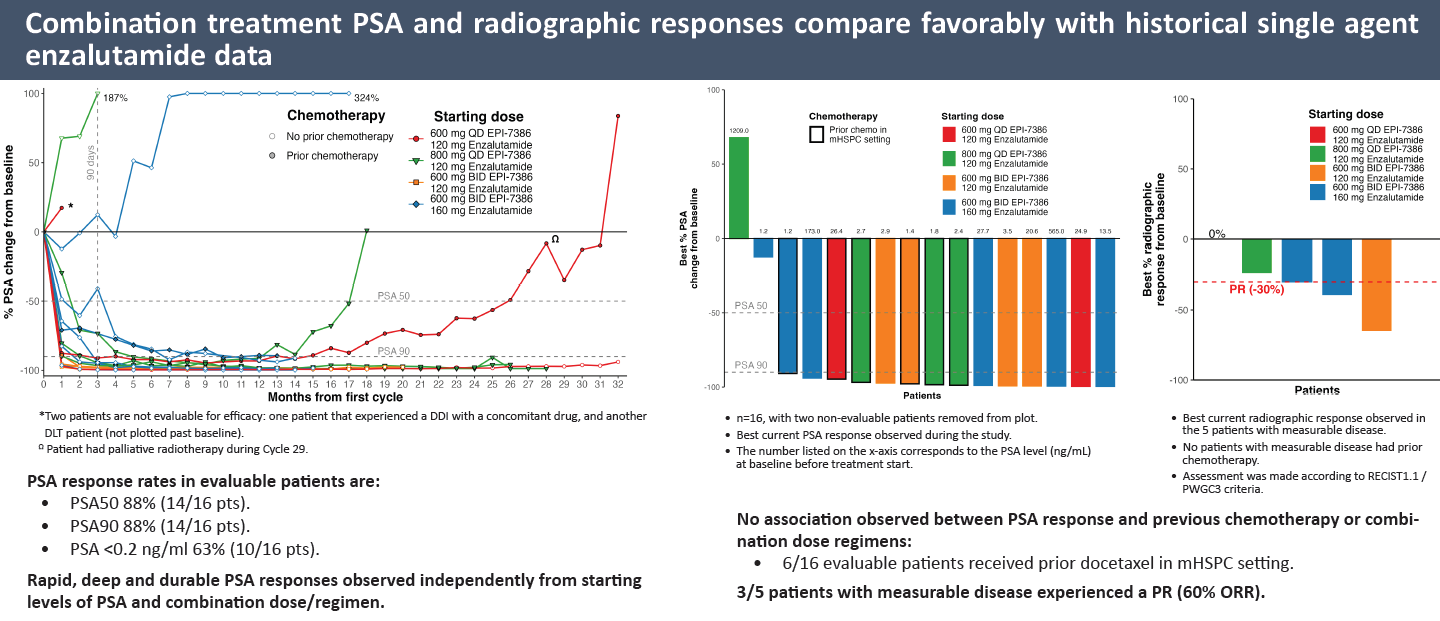
This seems a far cry from the promise expressed in September at ESMO, where dose-escalation data from this study were presented in a poster. These data concerned 16 evaluable patients, and trumpeted 88% PSA50 and PSA90 responses, with 11 patients achieving PSA90 in under 90 days.
Such responses, according to the poster, compared "favourably with historical single-agent Xtandi", but unfortunately no actual data for Xtandi monotherapy from the study were presented. In the event that comparison has proved to be crucial, as Essa now says single-agent Xtandi performed "better than historical controls".
With the phase 1/2 trial now futile, Essa has discontinued this as well as all other masofaniten studies, including combos with Zytiga, with Erleada and with Nubeqa. Essa claims still to be working on an unnamed third-generation N-terminal domain AR inhibitor, but this is still preclinical.
Interestingly, masofaniten isn't the company's first setback with N-terminal domain AR inhibition; Essa was earlier working on ralaniten, an early iteration of masofaniten, but this was discontinued in phase 1/2. Masofaniten was said to have 20-fold higher antiandrogenic potency than ralaniten, but even this hasn't been enough to beat tried and tested Xtandi.
603













