Ipsen casts doubt on Pfizer’s prostate plan
Mevrometostat is the only EZH2 inhibitor in phase 3 for prostate cancer.
Mevrometostat is the only EZH2 inhibitor in phase 3 for prostate cancer.

Pfizer took a bold step in moving its EZH2 inhibitor mevrometostat from phase 1 straight into phase 3 in castration-resistant prostate cancer, but months later the company might be nervously eyeing a low-key poster Ipsen presented on its contender Tazverik at ESMO last month.
That poster detailed the failure in the phase 1/2 Cello-1 study of Tazverik, the only approved EZH2 inhibitor, in the same prostate cancer setting, and in a similar combination with Xtandi. True, Pfizer might blame the molecule and not the mechanism, and has its own phase 1 success to point to, but whether EZH2 inhibition can move beyond relatively niche settings is still unclear.
Those niche settings comprise epithelioid sarcoma and follicular lymphoma, in which Tazverik holds accelerated US approvals. Ipsen gained Tazverik through the $245m acquisition of Epizyme in mid-2022, but in the most recent four quarters Tazverik sold $46m, and based on the ESMO poster expanding the drug into prostate cancer seems to be a dead end.
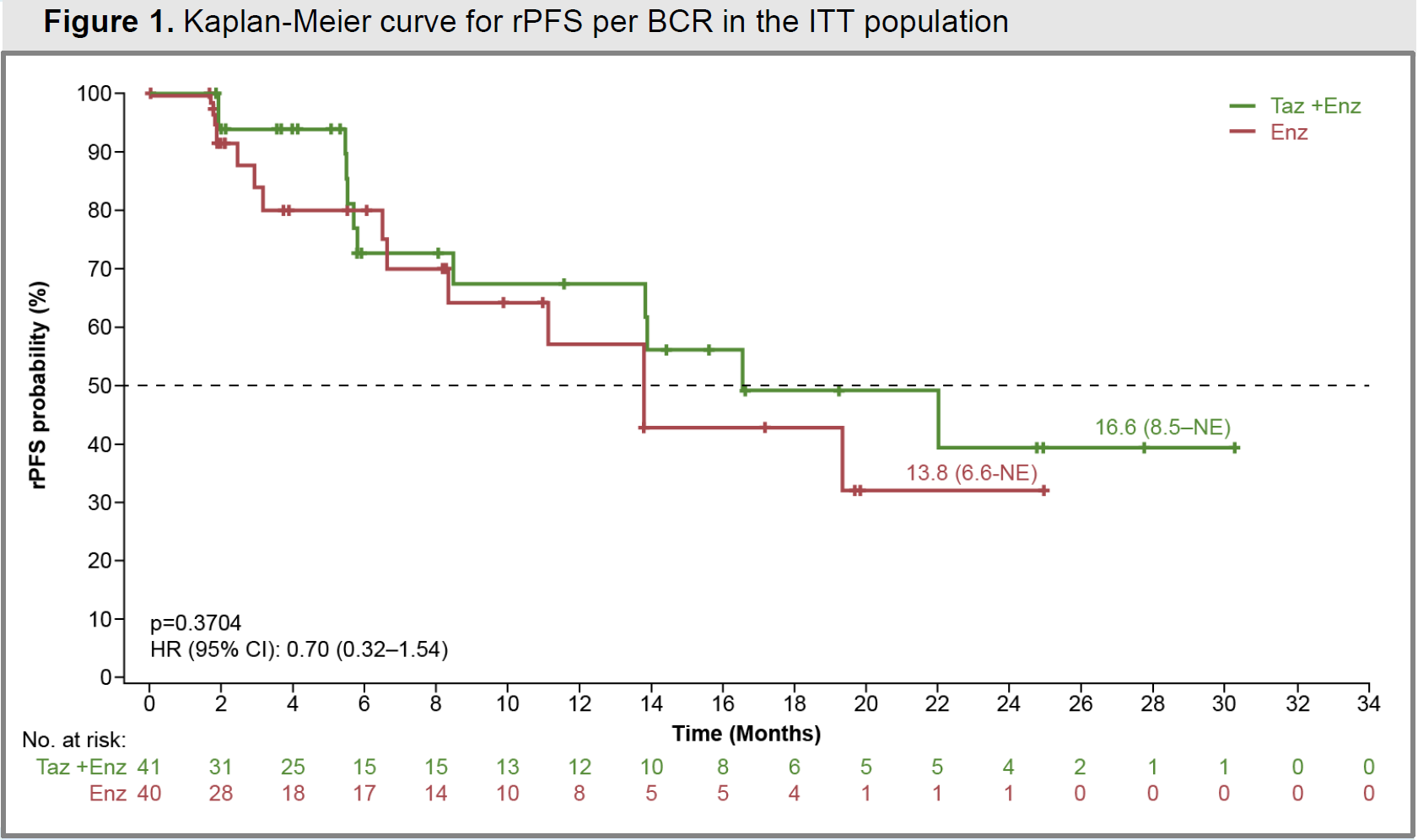
Cello-1 was a controlled trial, in which pre-chemo patients who had progressed on Zytiga were given Xtandi, with or without Tazverik. On the primary endpoint of radiographic PFS the combo scored a numerical benefit of 2.8 months at median, but the survival curves crossed over and there was not even a hint of statistical significance, with wide confidence intervals and a p value of 0.37.
Tazverik + Xtandi, vs Xtandi, in Cello-1
The rationale behind this approach is that the histone methyltransferase EZH2 can be seen in prostate cancer progression, and blocking EZH2 has preclinically been shown to reverse resistance to next-generation hormonal agents.
However, Cello-1 wasn’t the first trial to fail to replicate such findings clinically. For instance, Constellation Pharmaceuticals ran the phase 2 Prostar study, where post-Xtandi or Zytiga patients were given its EZH2 inhibitor CPI-1205 plus Zytiga or Xtandi respectively, and the latter group was compared against Zytiga progressers given Xtandi alone.
Prostar was discontinued in 2020 after failing to show a sufficiently strong signal to warrant pivotal development. Instead the company advanced a follow-on molecule, tulmimetostat, but this yielded no responses in prostate cancer in phase 2, and the project’s settings have been limited to haematology. In 2021 Constellation was acquired by Morphosys, which itself was bought by Novartis.
Jiangsu HengRui tested SHR2554 (later licensed to Treeline Biosciences) in a phase 1/2 prostate cancer study in which this EZH2 inhibitor was given alone or with androgen receptor inhibition. However, that study was terminated in 2021 after running for three years, with no data reported.
So what’s driving Pfizer’s enthusiasm? Most of all a phase 1 study of mevrometostat plus Xtandi, where median radiographic PFS was 17.1 months in post-Zytiga patients, and 11.7 months in those who had previously had Xtandi. The company argues that the number for the latter group would be around 4 months if given Xtandi alone, and on this cross-trial basis the 11.7-month number looks promising.
However, it’s the former setting of post-Zytiga patients that’s more relevant, and it’s here that Ipsen’s failed Cello-1 trial should give pause: mevrometostat’s 17.1-month median rPFS looks no different to the non-significant 16.6 months for Tazverik.
It’s precisely in this setting that Pfizer’s recently initiated Mevpro-1 study is now testing mevrometostat plus Xtandi versus Xtandi alone.
EZH2 inhibition in prostate cancer
| Project | Company | Status |
|---|---|---|
| Mevrometostat | Pfizer | Ph3 Mevpro-1 trial, Xtandi combo vs Xtandi in Zytiga progressers |
| XNW5004 | Evopoint Biosciences | Ph2 Xtandi combo in Zytiga progressers |
| Tulmimetostat | Novartis (ex Constellation (Morphosys)) | Ph2 solid tumour trial showed 6% ORR, including 0% ORR in 8 prostate cancer pts |
| SHR-2554 | Treeline Biosciences/ Jiangsu HengRui | Ph1/2 combo (incl Zytiga) ongoing; earlier ph1/2 prostate cancer trial terminated |
| Tazemetostat | Ipsen | Ph1/2 Xtandi combo showed no rPFS benefit vs Xtandi alone in Zytiga progressers |
| CPI-1205 | Novartis (ex Constellation (Morphosys)) | Discontinued after failing to show strong enough ph1/2 signal in prostate cancer |
| GSK2816126 | GSK | Discontinued after dose-limiting toxicity (liver enzyme elevation) in ph1 |
Source: OncologyPipeline.
2280













