No PRAME encore for Immatics
A 5% response rate with IMA402 monotherapy seems unlikely to turn heads.
A 5% response rate with IMA402 monotherapy seems unlikely to turn heads.

Judging by the first clinical data released on Immatics' anti-PRAME T-cell engaging MAb IMA402, the best that can be said about this company's attempts to build on the success of its lead project, IMA203, is that this is a work in progress.
IMA203 and IMA402 both target PRAME, but the former is an engineered T-cell receptor, and has so far yielded an impressive 55% response rate among PRAME-positive melanoma patients given a recommended phase 2 dose. On the back of this success Immatics took forward IMA402, but Monday's release of first clinical data for the latter project failed to live up to the promise of IMA203, and the group ended the day down 4%.
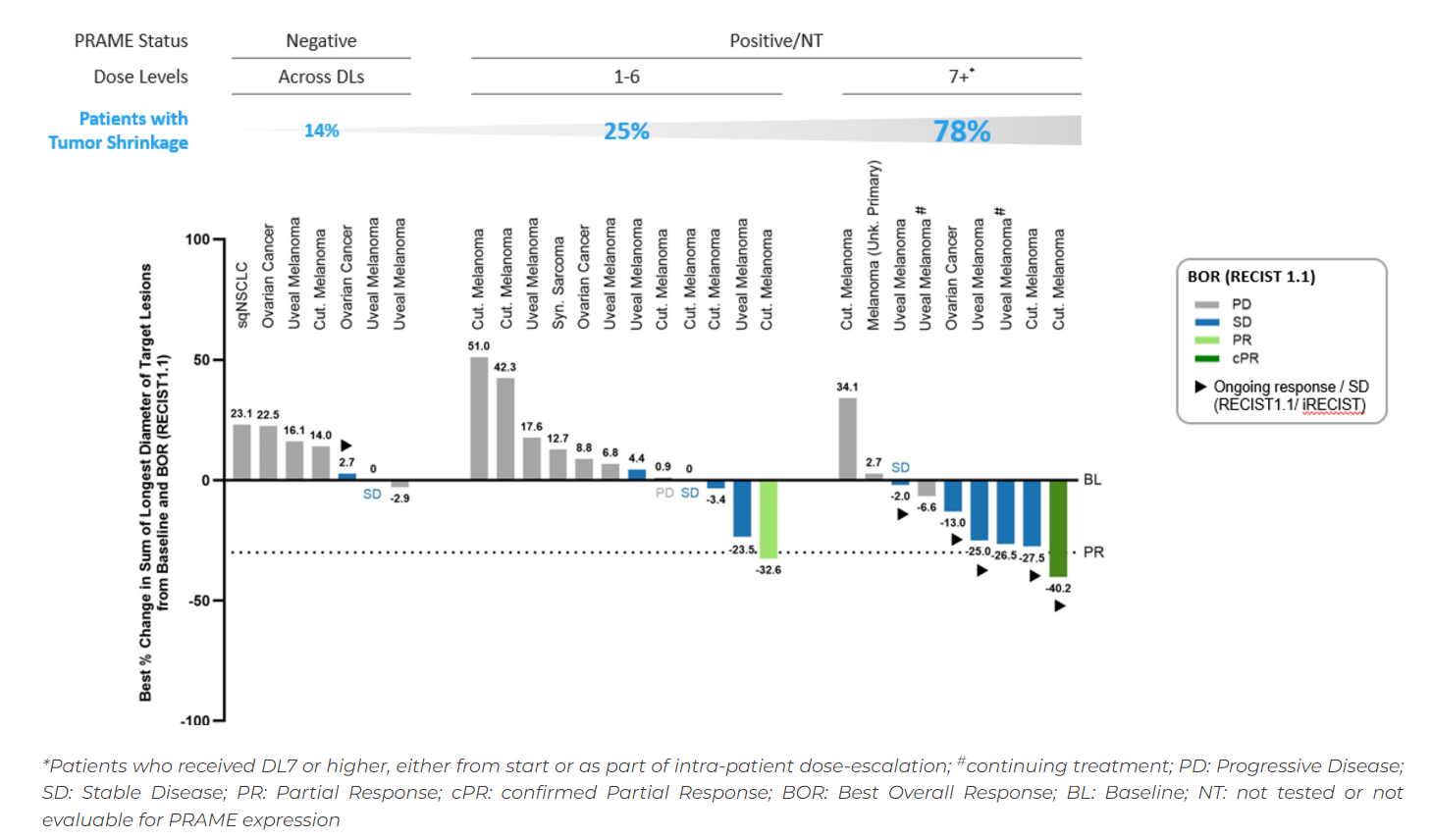
True, the IMA402 results are early, but they are unlikely to enthuse investors. Across 21 patients who weren't negative for PRAME, and of whom most had cutaneous or uveal melanoma, there were only two partial responses, and one of these relapsed before being confirmed. This puts the formal ORR among this group at a meagre 5%.
Caveats
This fact has left Immatics arguing that activity improved with increasing IMA402 doses, and with PRAME expression. Waterfalls provided as part of the company's third-quarter update do support this view, up to a point.
The greatest tumour shrinkage was seen among nine patients given dose level 7 or above, versus 12 subjects at dose levels 1-6. And, in seven patients documented to be non-PRAME expressing, there were no responses, with one stable disease and one marginal tumour shrinkage.
Overall this bodes well for higher dosing of IMA402, assuming that a sufficiently large therapeutic window remains open. Immatics said the most common adverse events were "mostly mild-to-moderate cytokine release syndrome and transient lymphopenia", and maximum tolerated dose hadn't been determined. However, it has had to implement step dosing.
The activity relationship with PRAME expression is less clear. Importantly, the 21 patients among whom the two responses were seen weren't necessarily PRAME-positive, but rather were either PRAME-positive or not evaluable for PRAME testing, or hadn't been tested for expression. Immatics provided no further detail as to whether the responders, and patients in whom tumours shrank, actually had confirmed PRAME expression.
IMA402 activity in phase 1
The data will be held up against Immunocore's brenetafusp, an anti-PRAME T-cell engager using a "soluble" T-cell receptor format that is now in a phase 3 Opdivo combo trial in melanoma.
Despite this the development of brenetafusp hasn't gone smoothly either, and this year's ASCO reported an 11% ORR among 36 melanoma patients given brenetafusp monotherapy. Brenetafusp has also shown activity in ovarian cancer, with a 23% ORR in 13 patients reported at ESMO; the best IMA402 has done in ovarian cancer so far is one stable disease among three patients.
Immatics is actually taking three shots at PRAME; in clinical trials it also has a next-generation T-cell receptor, IMA203CD8, which comprises IMA203 with an added CD8 co-receptor to allow CD4+ T cells to be utilised. At this year's SITC IMA203CD8 yielded a 41% confirmed ORR among 41 subjects, with one possibly treatment-related death and dose-limiting toxicities prompting a dosing change.
For Immatics it's full steam ahead with IMA203, which is to start the phase 3 Suprame trial next month. However, the early IMA402 data, plus Bristol Myers Squibb's recent termination of a deal covering an anti-MAGE-A4/8 T-cell receptor, raise questions about Immatics' ability to progress beyond its highly promising lead asset.
1909













