
ASCO-GI – early casdozokitug celebration for Coherus

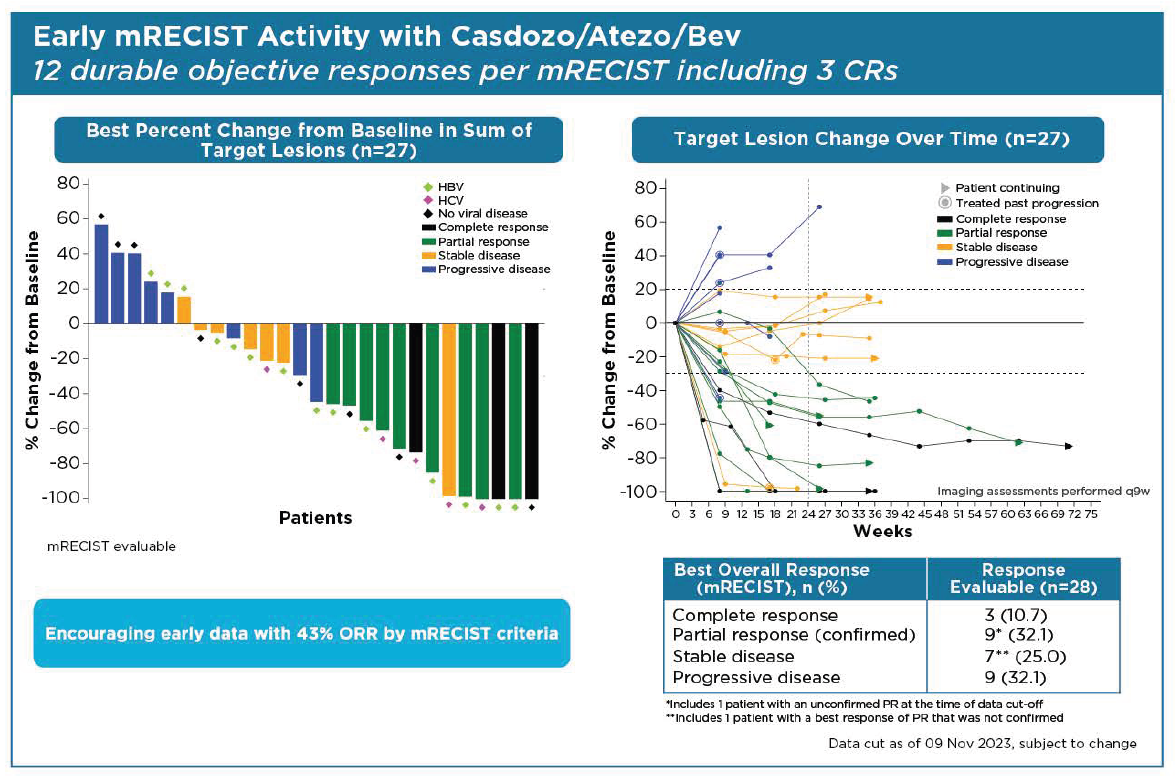
Fresh from reporting a qualified success with casdozokitug, Coherus has presented results at ASCO’s Gastrointestinal Cancers Symposium that it says back this anti-IL-27 agent’s promise as part of a triplet in first-line liver cancer. Close examination of the data, from the lead-in portion of a phase 2 trial, shows some reason for cheer, with the caveat of small patient numbers and the need for a cross-trial comparison. The results concern a triplet of casdozokitug, Avastin and Tecentriq that has yielded a best objective response rate of 38% or 43%, depending on the criteria used, in just under 30 patients. Comparing across trials, Avastin plus Tecentriq alone puts 33% of patients into remission, according to Imbrave-150, which backs the doublet’s first-line liver cancer approval. Coherus also cited median PFS of 8.1 months for the casdozokitug-containing triplet – again above the 6.8 months seen in Imbrave-150. Casdozokitug came as part of Coherus’s $65m all-stock acquisition of the distressed biotech Surface Oncology. Coherus today doubled down on oncology, selling an opthalmology biosimilars business to Sandoz for $170m, and says it’s now planning a liver cancer study combining casdozokitug, Avastin and its own approved anti-PD-1 MAb, Loqtorzi.
1397













