
Bayer changes tack in targeted lung cancer
As BAY2927088 enters phase 3 its lung cancer use will no longer be limited to exon 20.
As BAY2927088 enters phase 3 its lung cancer use will no longer be limited to exon 20.

As recently as last October Bayer was still describing BAY2927088 as an EGFR inhibitor with activity in lung cancer with exon 20 insertion mutations, but that’s apparently no longer its focus. The project is now said to have broader activity, and its first phase 3 trial, just revealed on the clinicaltrials.gov registry, will target HER2-mutated lung cancer.
This follows US breakthrough therapy designation, which the FDA in February gave BAY2927088 for NSCLC harbouring HER2 activating mutations, and a late-breaking poster on the phase 1/2 Soho-1 study in this use presented at ASCO. It’s possible that the shift was triggered by competitor developments, including the availability for exon 20 mutant NSCLC of Johnson & Johnson’s Rybrevant.
Rybrevant is a bispecific MAb that binds to EGFR and cMet, rather than a small molecule with activity on a specific mutated tyrosine kinase. But it’s carved out an important niche in NSCLC carrying EGFR exon 20 insertion mutations, while others have struggled: notably, Takeda had to withdraw its small-molecule drug Exkivity after this failed a confirmatory trial.
Unusual history
The history of BAY2927088 is somewhat unusual. An October 2022 paper described this small molecule as the first non-covalent tyrosine kinase inhibitor targeting EGFR exon 20 insertions and C797S resistance mutations in NSCLC.
At last year’s ESMO BAY2927088 was said also to have activity at EGFR exon 12 and 21 mutations, and Bayer presented data from the Soho-1 trial, which enrolled NSCLC patients with unspecified mutations in EGFR or HER2. Response rate was 26% across the 69-subject dataset, but the authors highlighted activity in 20 patients whose cancer was driven by exon 20 insertions in HER2: here ORR was 60%.
That was an impressive number, but HER2 exon 20 insertions are a relatively small niche, perhaps most notable for the failure of Spectrum’s poziotinib. Meanwhile, in EGFR exon 20 insertions the advance of Rybrevant and the withdrawal of Exkivity have been accompanied by small molecule discontinuations at Black Diamond and Blueprint.
Against this backdrop came Bayer’s breakthrough therapy designation and Soho-1 update at ASCO. The latter concerned an expansion cohort in NSCLC harbouring a HER2-activating mutation – including but not limited to exon 20 – in patients who hadn’t received an anti-HER2 therapy.
The headline number among these 33 subjects was a 70% ORR, with 15 of the 23 partial responses ongoing at a median 8 months’ follow-up. The vast majority of this dataset – 30 patients – comprised HER2 exon 20 insertions, but three subjects had a HER2 point mutation; a key detail is that two of these three patients had responses to BAY2927088.
BAY2927088 in the phase 1/2 Soho-1 study
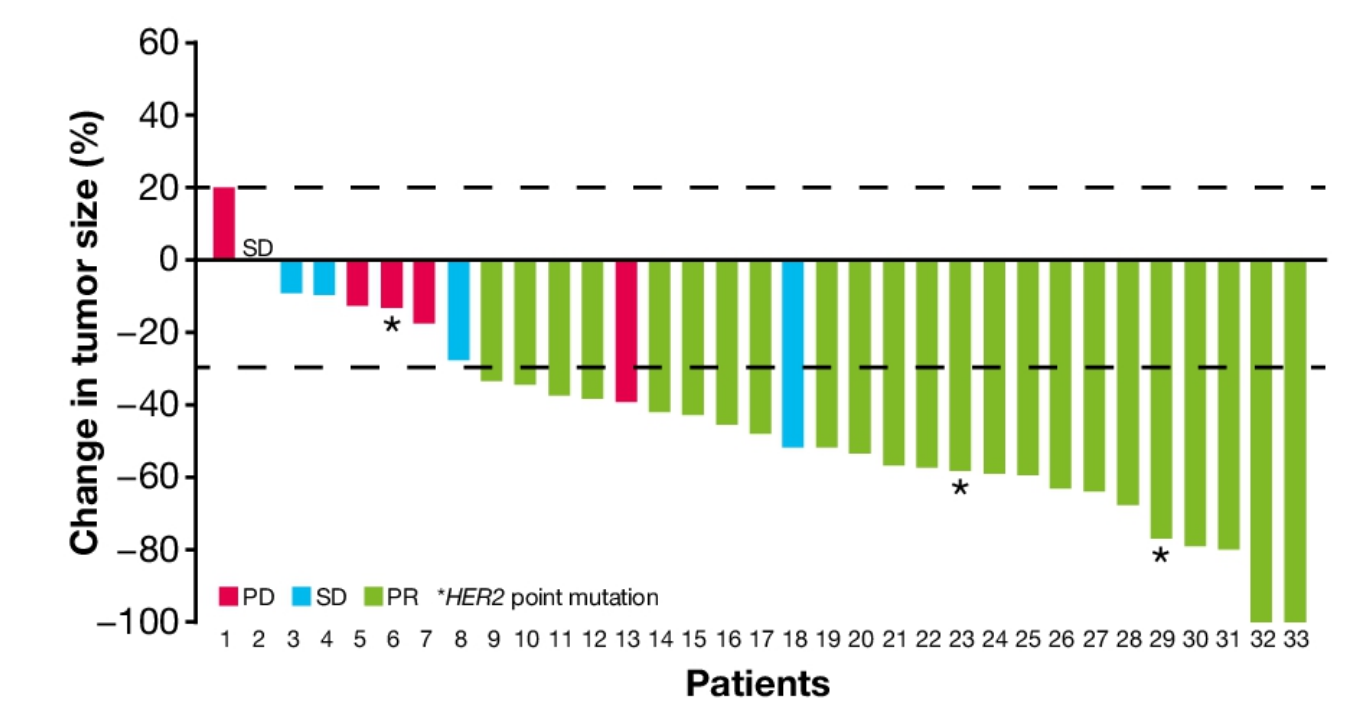
Now comes Bayer’s move into phase 3. The just revealed clinicaltrials.gov entry shows that this pivotal study will this month start enrolling 278 NSCLC patients with a documented activating HER2 mutation, and will primarily compare BAY2927088 versus Keytruda plus chemo on PFS; there is no need for patients to have exon 20-driven disease, but the first-line trial specifically precludes prior treatment with an exon 20-targeted therapy like poziotinib.
Given BAY2927088’s breakthrough therapy designation it’s possible that Bayer might file it for accelerated approval on the back of the Soho-1 data, and use the just listed phase 3 as the confirmatory trial. It will then be up to the FDA whether to limit the drug’s NSCLC use to exon 20 insertions or give it a broader HER2-mutant label.
1891













