
Nuvation lays out its US taletrectinib strategy

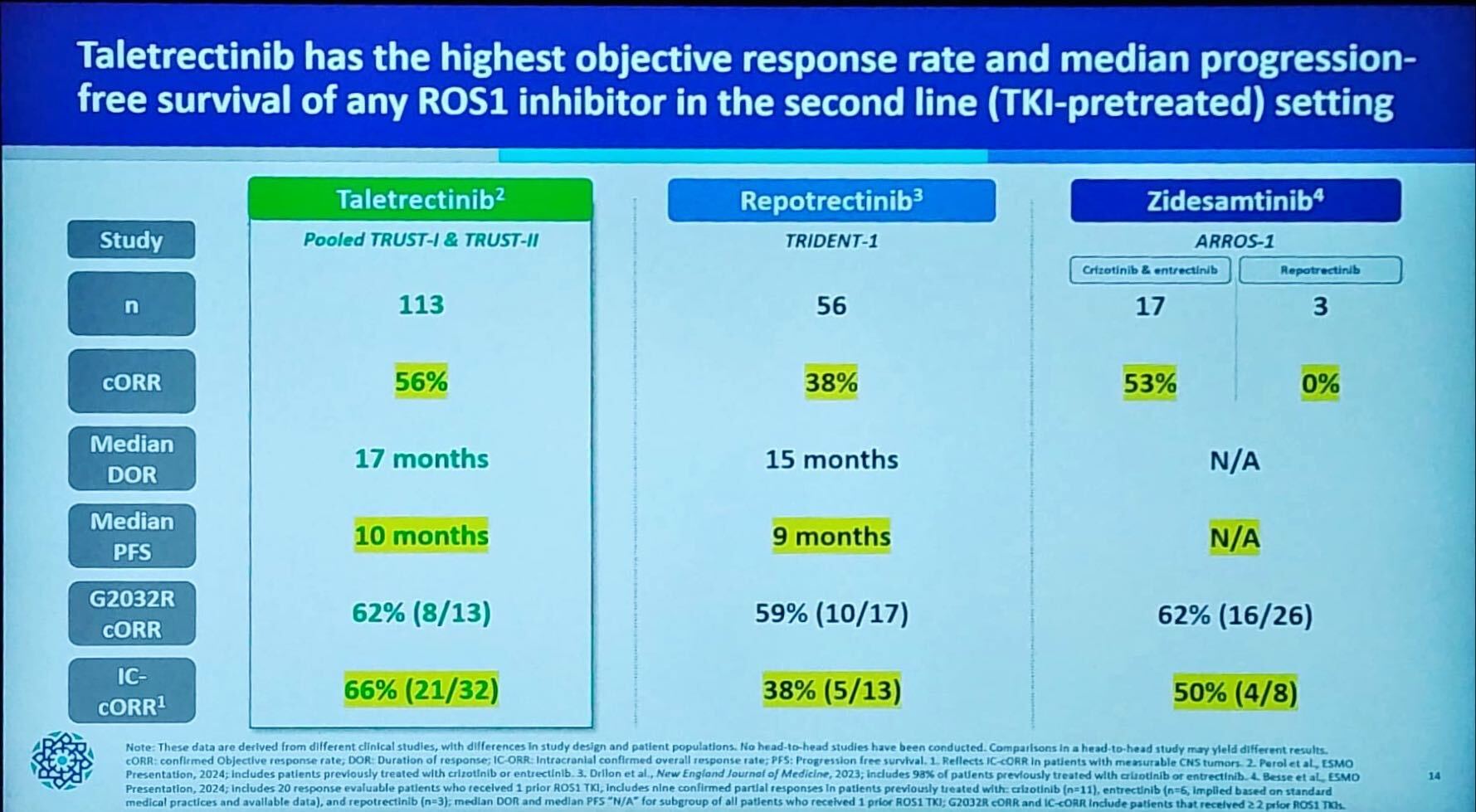
Nuvation recently started a phase 3 trial of its ALK/ROS1/NTRK inhibitor taletrectinib for Chinese regulators, but in the US it’s full steam ahead. The group confirmed at the Jefferies healthcare conference in London on Tuesday that it had filed the project with the FDA in ROS1-positive NSCLC, regardless of therapy line, based on pooled data from the phase 2 Trust-I and Trust-II trials. The group expects acceptance by year end, and approval in mid-2025. Nuvation’s chief executive, David Hung, noted that taletrectinib, which came via the acquisition of AnHeart, is the only ROS1 inhibitor with breakthrough designation, showing the FDA’s enthusiasm despite Trust-I being carried out in China; in Trust-II, meanwhile, only around 10% of patients were Chinese. Nuvation plans to launch taletrectinib itself in the US, but will look for a partner for Europe. The group would be up against Bristol Myers Squibb’s Augtyro, which is linked with CNS side effects and has so far produced unimpressive sales. Nuvalent’s zidesamtinib could prove a tougher opponent, but Hung presented a cross-trial comparison at Jefferies that, he contended, showed taletrectinib to be superior in the second line. Nuvation’s market cap is $885m, versus Nuvalent’s $6.2bn, suggesting that investors are less convinced.
44













