
ESMO 2024 – Keytruda/Lenvima leap... up to a point
Leap-012 delivers a technical win, but filing might hinge on overall survival and a call on toxicity.
Leap-012 delivers a technical win, but filing might hinge on overall survival and a call on toxicity.

The Leap-012 trial, in a non-metastatic liver cancer setting, has given Merck & Co and Eisai a rare pivotal win for a combination of Keytruda and Lenvima, late-breaking ESMO data showed on Saturday. Anyone hoping that this will lead to quick market introduction faces a big caveat, however: toxicity.
Little was known about Leap-012 until ESMO, and Merck/Eisai hadn’t even toplined the result as positive. In the study Lenvima plus Keytruda and transarterial chemoembolisation (TACE) beat TACE alone on PFS, but 71% of patients on active treatment suffered severe adverse events; this suggests why the companies are proceeding cautiously and likely awaiting OS data before filing.
Lenvima is known already to be associated with significant toxicity, and its prescribing information contains a long list of warnings from hypertension and cardiac, liver and kidney toxicity to osteonecrosis of the jaw. This explains why the toxicity seen in Leap-012 was described at ESMO’s presidential session as being “as expected”.
If Merck/Eisai are being cautious that’s warranted, and not only because of any questionable signs seen in Leap-012: virtually every other large study of Keytruda and Lenvima has failed, except the registrational Keynote-581 and 775 trials in front-line kidney and endometrial cancers respectively.
Leap-012 concerns intermediate-stage, non-metastatic liver cancer, and one of the combo’s flops has been Leap-002, in first-line unresectable liver cancer, where numerical OS and PFS benefits versus Lenvima alone didn’t yield a statistical win.
Bullish
Despite this, the tone at the ESMO presentation was bullish.
PFS was Leap-012’s primary endpoint, and on this measure Lenvima/Keytruda/TACE cut risk of progression or death by 34% (p=0.0002), with median PFS of 14.6 months versus 10.0 months for TACE alone. Presenting the data, Dr Josep Llovet from the University of Barcelona, called the PFS difference “profound”.
At this first interim analysis only 47.5% of the patients had died, but OS is showing a non-significant numerical benefit, with a 0.80 hazard ratio. Merck told ApexOnco that, while the PFS results were encouraging, Leap-012 was continuing to full OS readout, and that the company was discussing data with regulators; however, it hasn’t disclosed specific filing plans.
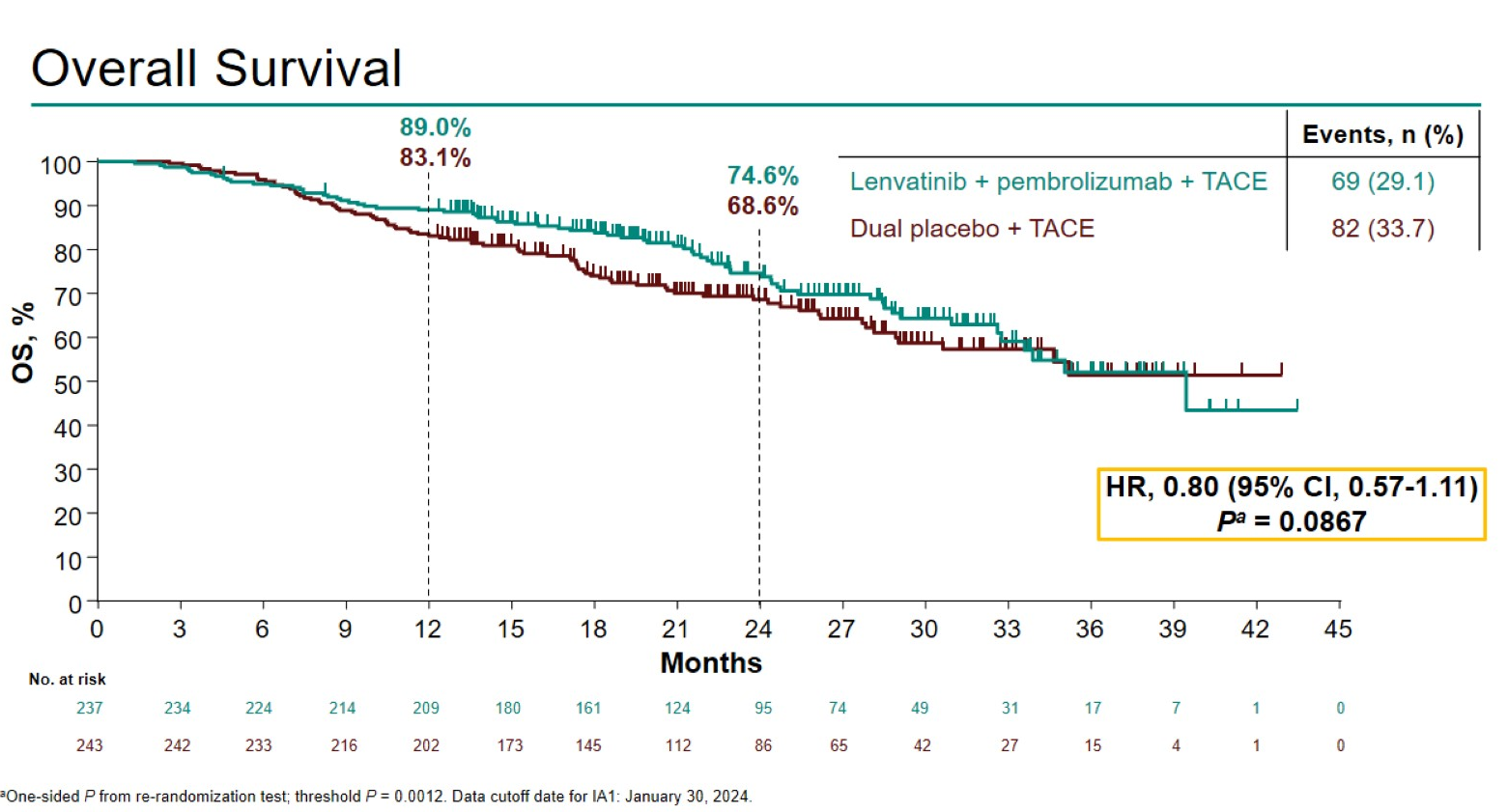
As for the toxicities, grade 3-5 treatment-related adverse events occurred in 71.3% of patients in the Lenvima/Keytruda/TACE group versus 31.5% for patients given TACE alone. There were four treatment-related deaths in the active group, from liver failure, GI haemorrhage, myositis and immune-related hepatitis, while one control patient died from brain stem haemorrhage.
TRAEs led to treatment discontinuation in 8.4% versus 1.2% of TACE-only patients. Liver enzyme elevations were seen in around 35% of Lenvima/Keytruda/TACE patients, including some 5% at grade 3 or 4, but the latter didn’t appear meaningfully different versus control.
The tone of the discussant, Angela Lamarca from University Hospital, Madrid was also upbeat. Asked whether safety was acceptable she said “I think so”, adding that she was optimistic about a future OS hit. Would she accept the Lenvima triple therapy as a new standard purely on the basis of PFS, without a mature OS benefit? “Yes, probably,” she told ESMO.
One reason for her optimism was the lack of other novel drugs in this non-metastatic liver cancer TACE combo setting. The only comparable trial is the Emerald-1 study of AstraZeneca’s Imfinzi plus TACE and Avastin, but here only a PFS benefit versus TACE has been shown, with nothing said about OS, and the PFS curves separated later than in Leap-012, Lamarca said.
Moreover, the Emerald-1 result appeared to have been driven by Avastin, rather than by Astra’s anti-PD-L1. Like Merck, Astra says it’s awaiting OS data, and its filing plans are unclear.
1188













