
ASCO-GI – Braftovi shoots for a survival benefit

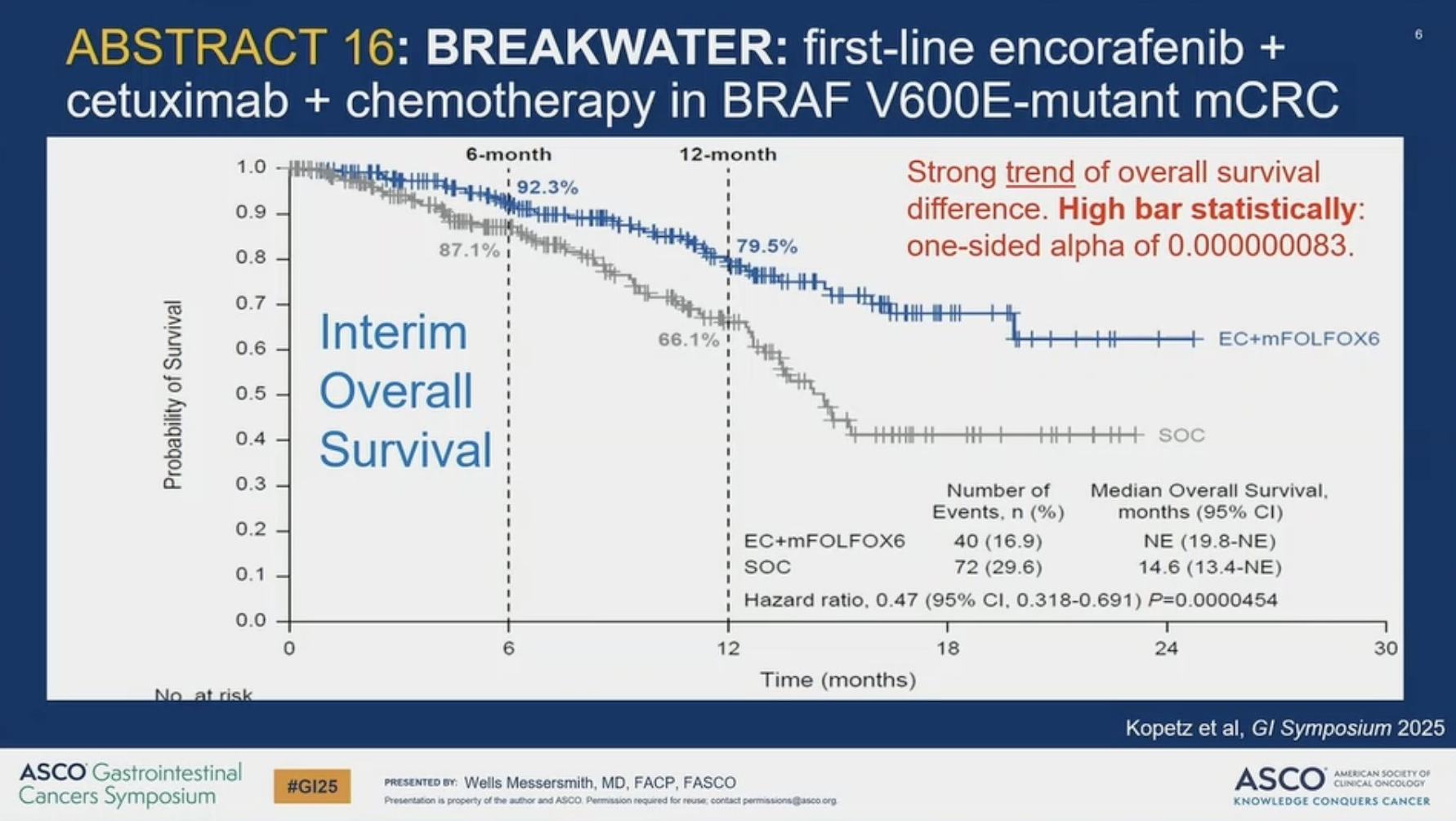
Pfizer's Braftovi, fresh from its US accelerated approval as part of an Erbitux and chemo combination for first-line BRAF-mutated colorectal cancer, might be heading for a full FDA green light. That's because its supporting Breakwater study, which also serves as the accelerated approval's confirmatory trial, is already showing a strong separation in overall survival curves. The ORR data were presented over the weekend at the ASCO Gastrointestinal Cancers symposium, but have little novelty value, having already been disclosed in Braftovi's updated US prescribing information. Of much more interest was a first look at OS: with 16.9% of patients in the treatment arm having died the study is showing a 53% reduction in risk of death versus standard of care. This extremely early look at the data naturally features extensive censoring, but has already yielded a p value of 0.00005, though for a statistical win to have been declared at this point a one-sided p of 0.00000008 would have to have been cleared. University of Colorado's Dr Wells Messersmith, discussing the data at ASCO-GI, called this a strong OS trend, but cautioned about Breakwater's high rates of treatment discontinuation, dose reduction and interruption.
748













