ESMO 2023 – how datopotamab's lung side effects are graded

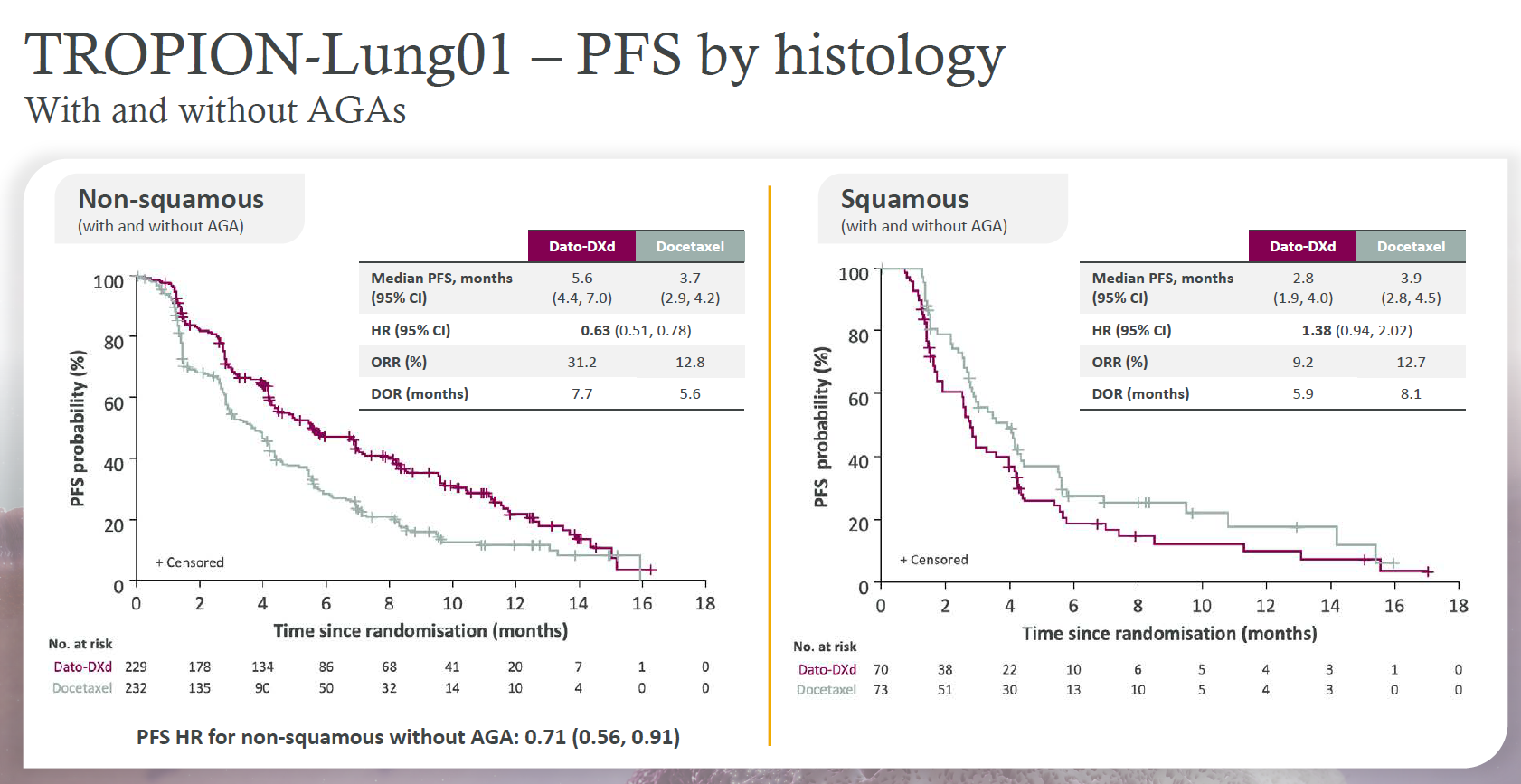
Interstitial lung disease, a running sore in the development of Daiichi Sankyo/AstraZeneca’s antibody-drug conjugates, remained a topic of discussion at yesterday’s ESMO presidential presentations, and the way such events are graded could make it tricky to interpret their importance in future trials. Datopotamab deruxtecan’s Tropion-Lung01 and Tropion-Breast01 studies saw seven and one patients respectively develop centrally adjudicated drug-related grade 5 interstitial lung disease (ILD) after getting datopotamab, it was revealed. The ILD data were adjudicated by a central, independent committee, and in the former study all seven appear under adverse events even though the primary investigator on site in four of them attributed primary cause of death to disease progression. In Tropion-Breast01, meanwhile, the one centrally adjudicated drug-related grade 5 ILD was ultimately graded 3, and secondary to disease progression, by the investigator. On the efficacy side it became clear that dato had no future in squamous NSCLC after a subgroup analysis showed a 1.38 hazard ratio for PFS in these patients in Tropion-Lung01, with a numerical benefit favouring docetaxel control. At yesterday's ESMO press conference the discussant, Memorial Sloan Kettering’s Dr Sarat Chandarlapaty, said the result showed dato to be a meaningful second-line treatment only for patients with the non-squamous histology.
1866













