
SGO 2025 – Genmab’s advantage looks Profound
While Sutro shows why it’s shelved luvelta-T.
While Sutro shows why it’s shelved luvelta-T.

Genmab needed good news after last week’s failure of its next-generation Darzalex, and it got some on Monday with updated results from its ProfoundBio-originated ADC rinatabart sesutecan. The latest ovarian cancer data from the Rainfol-01 study, presented at the Society of Gynecologic Oncology meeting, show response rates with rina-S improving since last year’s ESMO: ORR at the go-forward dose is now 56%, up from 50%.
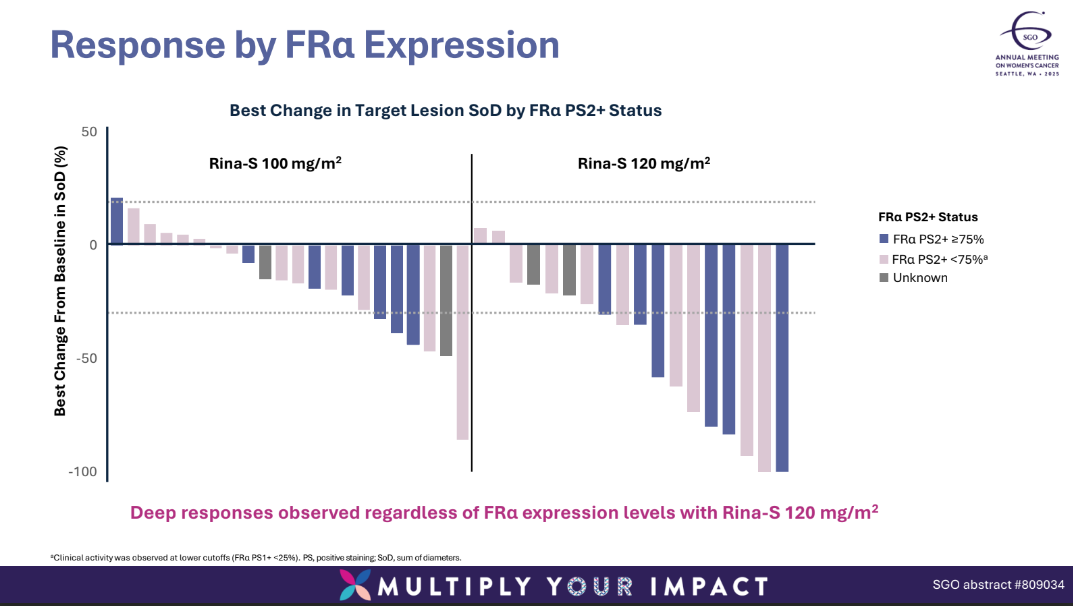
SGO also neatly demonstrated why Genmab plumped for ProfoundBio over its folate receptor alpha rival Sutro, which last week deprioritised its contender, luveltamab tazevibulin. On Saturday SGO heard that the most effective dose of luvelta-T produced an ORR of just 32% in platinum-resistant ovarian cancer in the Refrαme-O1 study – which notably only enrolled patients with FRα expression levels of 25% or higher.
Genmab, meanwhile, claimed responses in patients regardless of FRα expression levels.
Make it rain
Rainfol-01, an uncontrolled phase 1/2 trial, enrolled patients with various solid tumours known to express FRα; levels of the biomarker were retrospectively assessed. Cohort B1, which was presented at SGO, focused on advanced ovarian cancer; patients had received a median three prior therapy lines, and around 90% were platinum resistant. They received rina-S dosed at 100mg/m2 or 120mg/m2 every three weeks.
Genmab had already decided to take the 120mg dose forward, and it’s clear to see why, with response rates much better higher but without an apparent increase in toxicity.
Evolving Rainfol-01 cohort B1 results
SGO 2025 | ESMO 2024 | |
|---|---|---|
| Cutoff date | 15 Jan 2025 | 28 Jul 2024 |
| Confirmed ORR | ||
| - 120mg/m2 Q3W | 56% (10/18) | 50% (9/18) |
| - 100mg/m2 Q3W | 23% (5/22) | 18% (4/22) |
| ≥Gr3 TEAEs | ||
| - 120mg/m2 Q3W | 65% | 60% |
| - 100mg/m2 Q3W | 73% | 64% |
Source: conference presentations.
Adverse events to keep an eye on will be anaemia and neutropenia, seen in 45% of patients on the 120mg dose at grade 3 or higher.
There were also discontinuations, due to liver enzyme elevations and neutrophil count decrease, which were deemed treatment related. Two deaths occurred, one in each dosing cohort, but these were classified as unrelated to rina-S. Genmab noted no signals of ocular toxicity, neuropathy or interstitial lung disease.
While promising, these results will need to be replicated in more patients in a controlled trial, if Genmab is to fulfil its goal of making rina-S a $2bn-plus drug.
Genmab's claim of activity across FRα expression levels will also need to hold up. The SGO presentation merely details responses at levels above and below 75%, although it also notes that clinical activity was seen at levels below 25%.
The company has already begun the pivotal Rainfol-02 trial, in second-line-plus platinum-resistant ovarian cancer, with data expected in 2026.
There are also more results to come from Rainfol-01, including from cohort B2 in endometrial cancer, due in the first half. Genmab intends to start a phase 3 study in second-line endometrial cancer this year, and also has pivotal plans in first-line disease.
Meanwhile, Sutro’s luvelta-T continues to look like an also ran. The 32% ORR in the phase 2/3 Refrαme-O1 trial, with a 5.2mg/kg dose, is in line with data released in December, which itself was a disappointment after the phase 1 STRO-002-GM2 study found an ORR of 44% among a subgroup of patients with ≥25% FRα expression.
Sutro is trying to find a licensing partner for luvelta-T; however, on the basis of this evidence it might have trouble drawing interest.
This story has been updated in light of the full SGO presentation.
1612













