
Pfizer makes haste to catch up in PD-L1

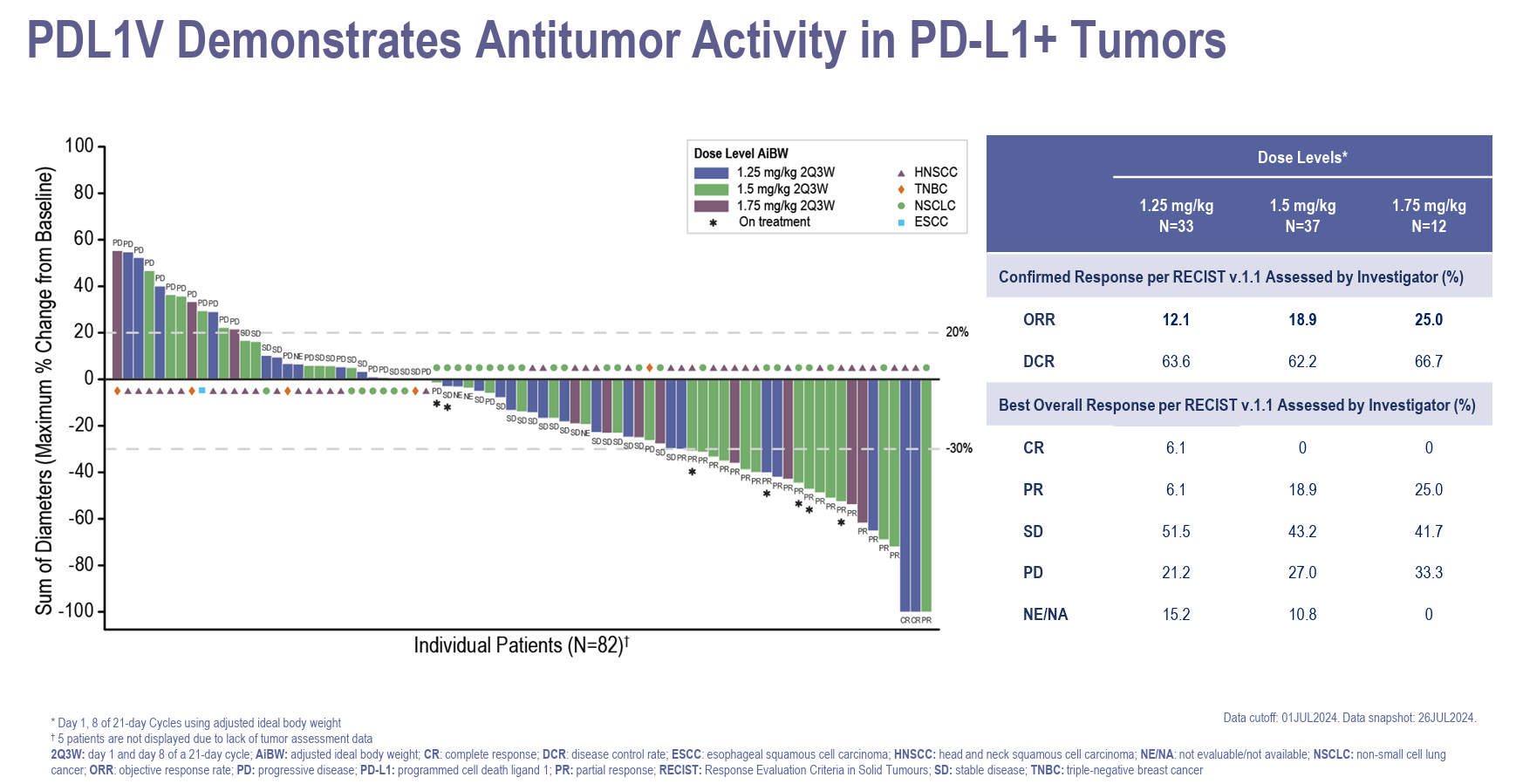
Just one year after Pfizer highlighted PF-08046054 as an early pipeline asset to watch, this anti-PD-L1 ADC is heading for phase 3. During Tuesday's full-year earnings presentation the company said 2025 would see the start of two pivotal studies, one in first-line head and neck cancer, and the other in relapsed non-small cell lung cancer. The key backing for this move appear to be data presented at last year's ESMO, where in a phase 1 trial in PD-L1-positive solid tumours there was a 17% response rate among 82 patients on the highest three doses. Among head and neck and NSCLC patients investigator-assessed ORRs for these doses were 16% and 22% respectively. It's likely that Pfizer is conscious of its failure to get an anti-PD-(L)1 antibody across the regulatory finish line, and perhaps because of this the group is keen to make haste in taking a first-in-class anti-PD-L1 ADC to market. PF-08046054 was originated by Seagen, and carries an MMAE (vedotin) payload; Pfizer's pipeline includes a related asset, PF-08046037, whose payload is a TLR7 agonist, but this is still preclinical. According to OncologyPipeline these two molecules are the industry's only anti-PD-L1 ADCs.
2191













