
MAIA throws more money at ateganosine
The THIO-104 study listing is live, along with questions about funding the trial.
The THIO-104 study listing is live, along with questions about funding the trial.

MAIA Biotechnology has become the latest biotech to push into phase 3 despite dwindling cash. A recent listing on clinicaltrials.gov shows a pivotal trial of its telomere-targeting project ateganosine set to begin in July.
If all goes to plan the new study, THIO-104, in third-line NSCLC, could confirm a possible future accelerated approval based on the ongoing phase 2 THIO-101 study. But MAIA has a long way to go – the group has only just begun the registrational portion of THIO-101, and there are questions about whether it has sufficient funds for a pivotal push.
At the end of last year the company had just $9.6m in the bank. Since then it has carried out a couple of private placements, but these only brought in a total of $4.1bn – which MAIA said was earmarked for starting the final part of THIO-101. Notably, the FDA has tended no to grant an accelerated approval in the absence of a confirmatory trial being substantially enrolled.
As such there seems a possibility that MAIA will begin a phase 3 trial it’s ultimately unable to complete. The company isn’t the only one in this position, with cash-strapped Imunon recently revealing phase 3 plans for IMNN-001, and Tempest throwing in the towel before starting recruitment into a pivotal trial of its contender amezalpat.
The THIO-104 trial is set to test ateganosine (formerly known as THIO) followed by Libtayo, versus investigator’s choice of chemo, in third-line NSCLC patients resistant to checkpoint inhibitors. The primary endpoint is overall survival.
The group has set out its expectations for THIO-104, along with the pivotal portion of THIO-101, which is also evaluating ateganosine followed by Libtayo, but with a primary endpoint of ORR. That trial also includes an ateganosine monotherapy arm.
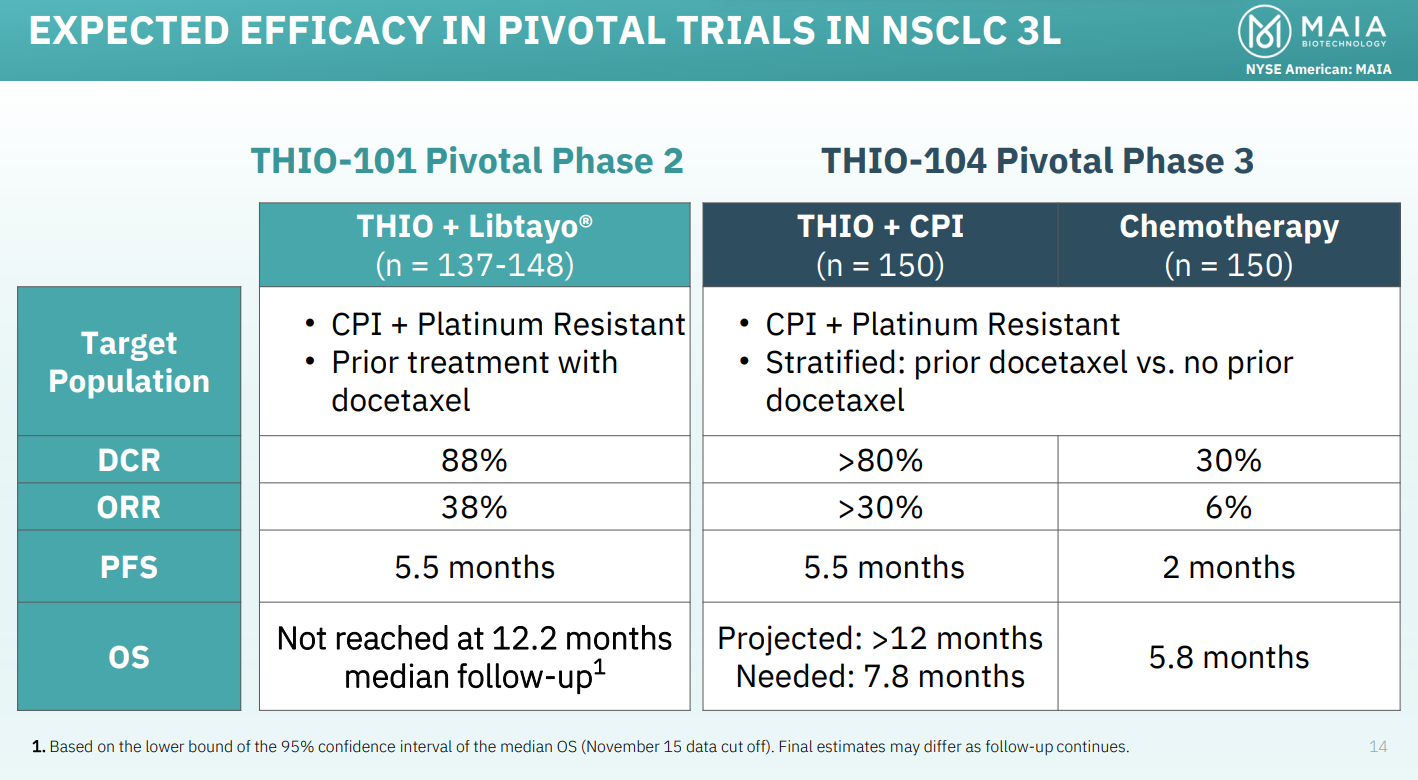
THIO-101 has so far completed safety and dose-selection evaluation, where it showed a median overall survival of 16.9 months among 22 patients, according to MAIA.
The company hopes to get accelerated approval of ateganosine in 2026, based on THIO-101, adding that THIO-104 could support “early full approval”, based on an interim analysis, later that year.
It also has plans for ateganosine in other indications, with the THIO-102 basket trial and THIO-103 in first-line lung cancer both slated to start next year. Raising more money should be it first priority.
Notable trials of ateganosine
| Trial | Phase | Setting | Details | Timing |
|---|---|---|---|---|
| THIO-101* | 2 | 3rd-line NSCLC (checkpoint inhibitor resistant) | Ateganosine +/- Libtayo | Enrolling Q1 2025; MAIA hopes for accelerated approval 2026 |
| THIO-104 (confirmatory trial) | 3 | 3rd-line NSCLC (checkpoint inhibitor resistant) | Ateganosine + Libtayo, vs investigator’s choice chemo | To start Jul 2025 |
| THIO-102 | 2 | Basket trial in CRC, HCC & SCLC | Ateganosine + checkpoint inhibitor | Planned to start early 2026 |
| THIO-103 | 2/3 | 1st-line NSCLC & SCLC | Ateganosine + checkpoint inhibitor | Planned to start 2026 |
Note: *details given for registrational portion in 3L NSCLC. Source: OncologyPipeline & clinicaltrials.gov.
125













