
Keros challenges Geron

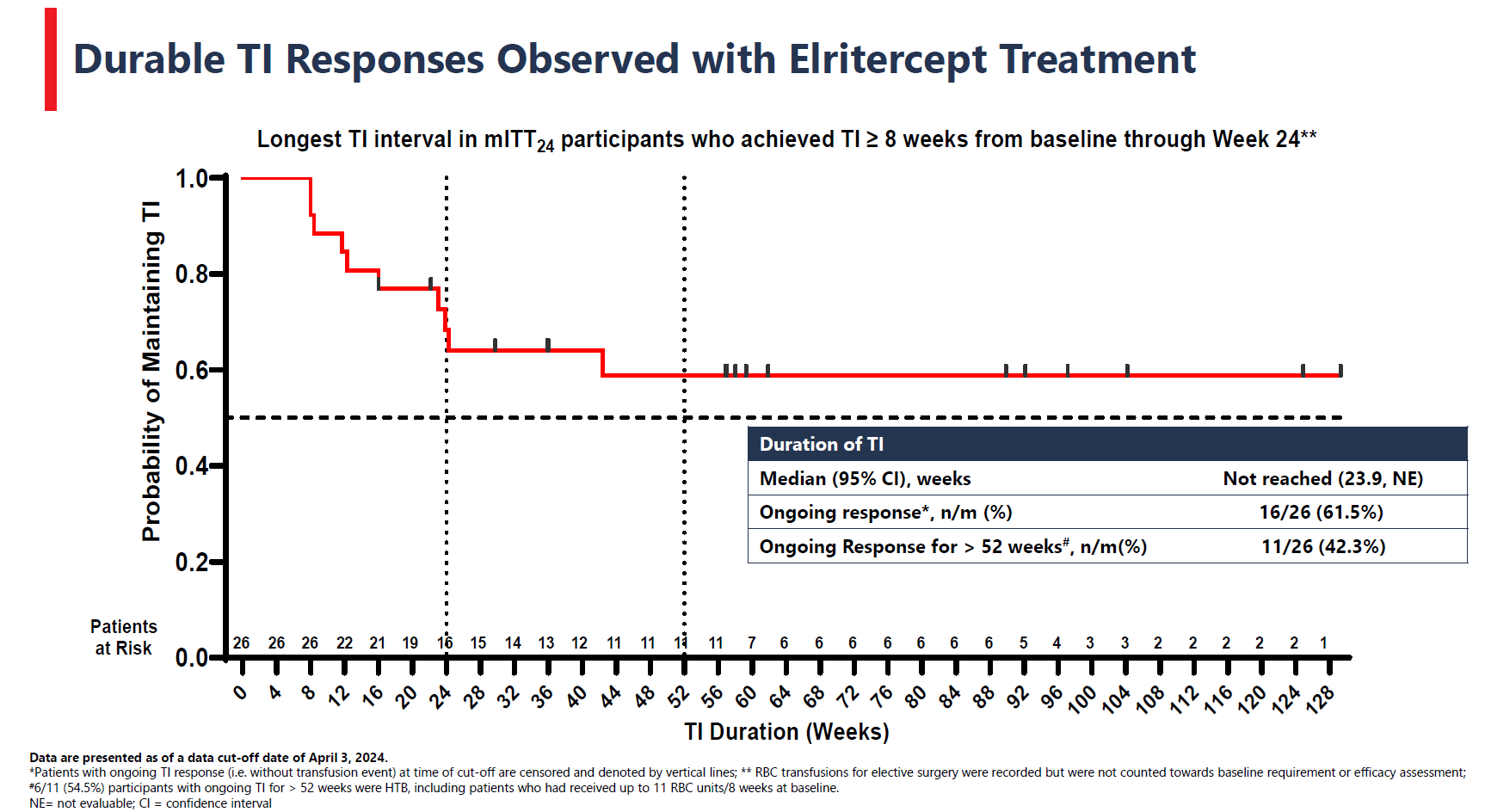
Shortly after Keros Therapeutics’s lead project, elritercept, was said to show a continued positive effect in low-risk myelodysplastic syndromes in a mid-stage trial, the company hopes to move this recombinant fusion protein into phase 3 in this patient population. A just published clinicaltrials.gov entry reveals the pivotal Renew study, in 225 patients refractory, intolerant or unlikely to respond to erythropoiesis-stimulating agents (ESAs), measuring eight-week transfusion independence versus placebo (2:1 randomisation) as primary endpoint. However, Renew isn’t expected to start until the end of December. Keros claims to specialise in disorders linked to TGF-β dysfunction, and elritercept comprises a modified ligand-binding domain of TGF-β receptor activin receptor type IIA (ActRIIA) linked to an Fc domain. One consideration here is Bristol Myers Squibb’s Reblozyl, a TGF-β ligand trap approved for treating low-risk MDS after ESAs – but only in patients with so-called ringed sideroblasts. Renew forbids prior Reblozyl use, and how patients without ringed sideroblasts do on elritercept will be of note. That’s one supposed advantage of Geron’s recently approved Rytelo, a telomerase inhibitor that can be prescribed for post-ESA low-risk MDS patients irrespective of ringed sideroblast status, so Renew is a clear challenge to Rytelo’s standing here.
702













