Cautious support for targeting CDH6

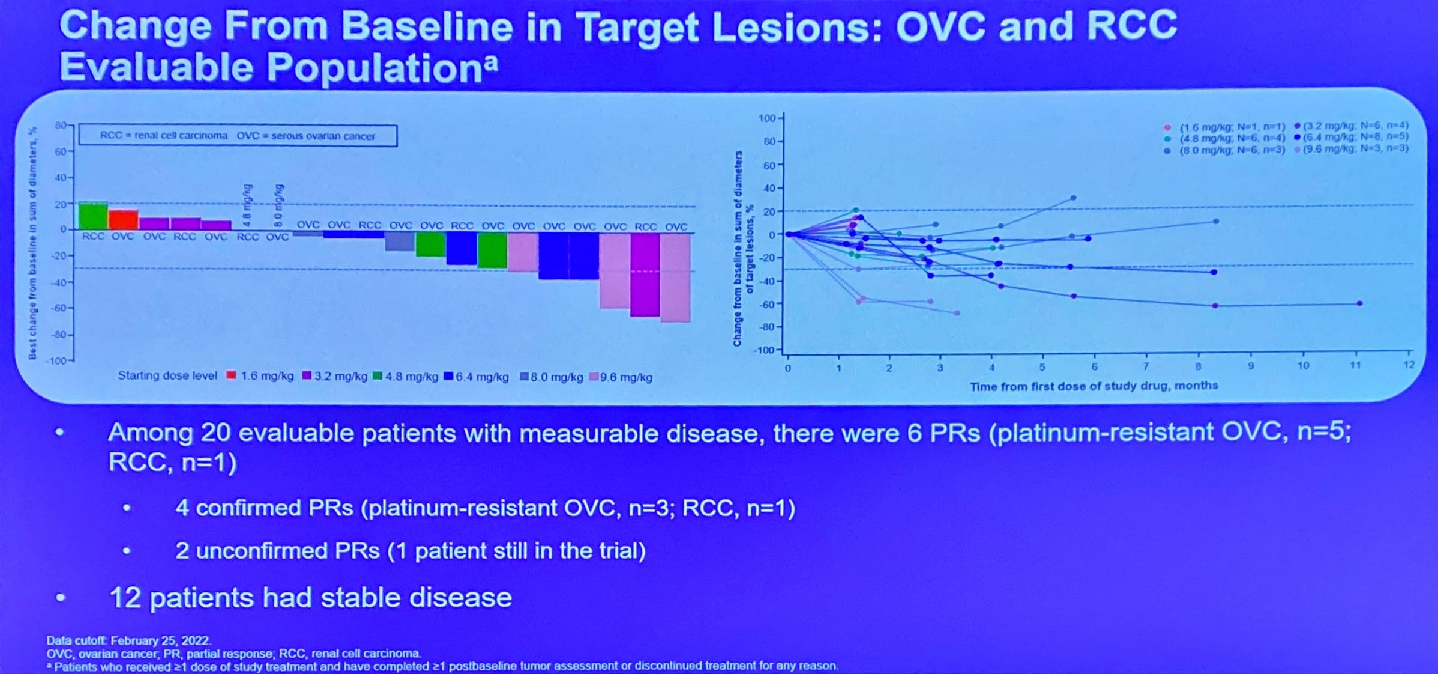
One year after licensing Multitude Therapeutics’ AMT-707/CUSP06, the private US biotech OnCusp has received clearance to start a phase 1 trial. This has yet to appear on clinicaltrials.gov, but will assess the safety and tolerability of escalating doses in patients with platinum-refractory/resistant ovarian cancer and other solid tumours. CUSP06 is an ADC targeting CDH6, so it will be key to see the extent to which any efficacy correlates with the expression of this antigen on patients’ cancers. The development is especially interesting because there is hardly any competing work in CDH6 targeting, and the lead asset is Daiichi Sankyo's DS-6000, an asset not included in the Japanese group's AstraZeneca alliance that has given rise to Enhertu. Daiichi had once called DS-6000 a rising star, but has not said much about it since presenting first-in-human data at ASCO 2022 showing six partial remissions (two unconfirmed) among 20 patients. OnCusp says CUSP06 includes a protease-cleavable linker and exatecan payload enabling “bystander” activity, with a drug-to-antibody ratio of around eight. The fact Novartis’s anti-CDH6 ADC HKT288 was scuppered by toxicity not long ago might weigh heavy for Daiichi and OnCusp alike.
DS-6000 data at ASCO 2022
Industry projects targeting CDH6
| Project | Companies | Modality | Status |
|---|---|---|---|
| DS-6000 | Daiichi Sankyo | ADC | Ph1 in renal cell and other solid tumours |
| CUSP06 | OnCusp/Multitude Therapeutics | ADC | IND cleared to start ph1 in ovarian and other solid tumours |
| HKT288 | Novartis | ADC | Discontinued in ph1 (neurotoxicity) |
| NeoPep GT | CureBio | GRS peptide | Discontinued in preclinical |
Source: OncologyPipeline.
505