
ASCO-GU – Opdivo's approval might have scuppered Keynote-123
The Bristol drug's availability for adjuvant bladder cancer threw Keynote-123 out of kilter.
The Bristol drug's availability for adjuvant bladder cancer threw Keynote-123 out of kilter.

Merck & Co’s attempt to extend Keytruda’s urothelial bladder cancer position, recently boosted by the approval of a first-line Padcev combo, might have hit a snag.
Late-breaking data unveiled at ASCO’s Genitourinary Cancers Symposium from the Keynote-123 study of Keytruda in the adjuvant setting on Friday confirmed a favourable interim result on disease-free survival versus observation, but not on its important co-primary endpoint of overall survival. US approval of Opdivo in this setting, likely leading to a high rate of patient withdrawals before disease progression in Keynote-123, suggest a possible explanation.
At first glance the overall survival data look especially bad given that medians numerically favour observation, at 55.8 months, versus 50.9 months for Keytruda. But investigators highlighted the fact that 17% of of active cohort patients, and 27% in the control group, withdrew consent without experiencing disease progression, leading to uneven censoring in Keynote-123’s analysis.
In addition to patients who withdrew consent, 5% of those on observation received a checkpoint inhibitor before disease progression, and another 22% after.
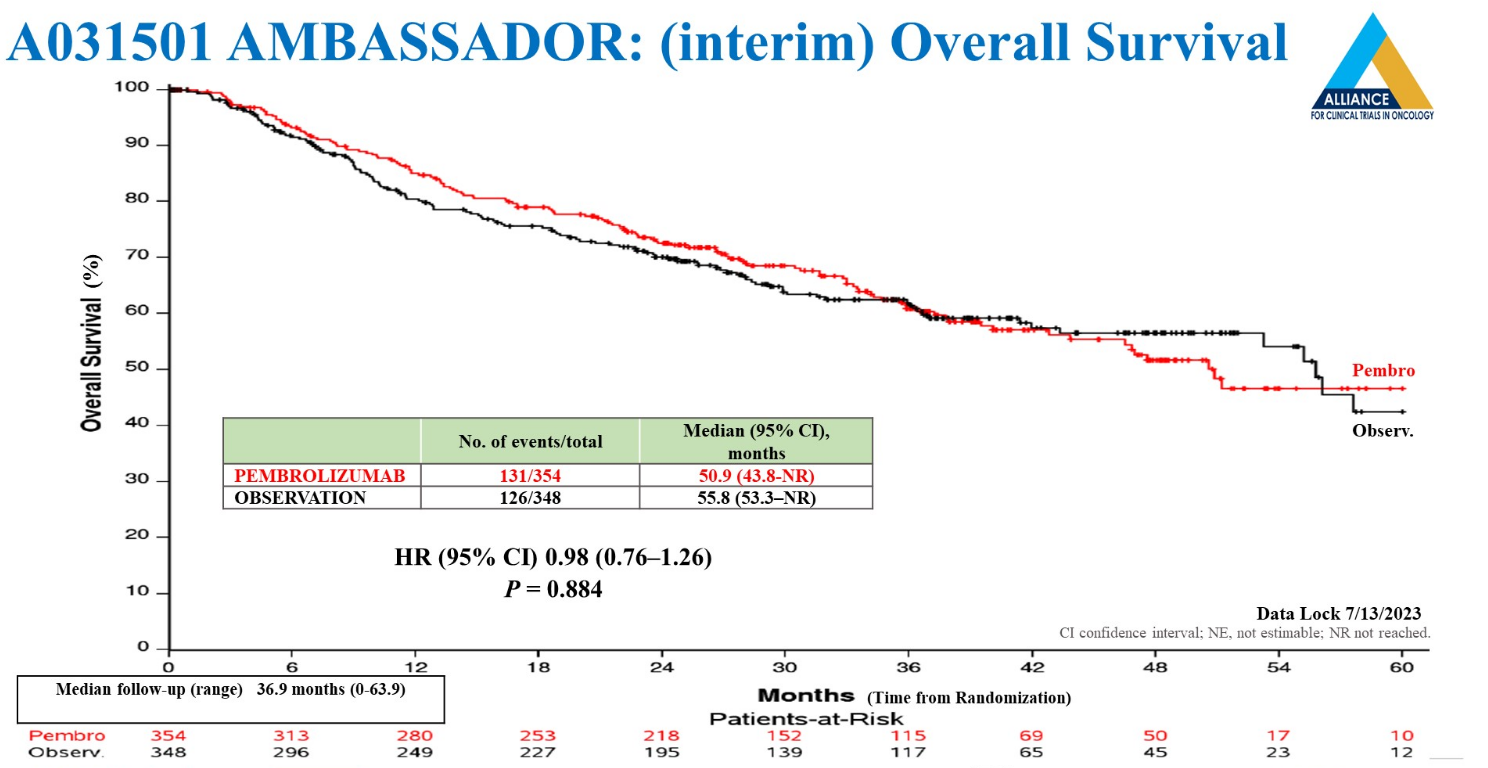
This points in one direction, namely the availability, since August 2021, of Bristol Myers Squibb’s Opdivo for adjuvant treatment of bladder cancer, which also caused Keynote-123 to be closed early, with accrual 4% short of the planned 734 patients.
The effect of Bristol’s approval is that it turned Keynote-123’s OS analysis in part into a comparison of Keytruda versus Opdivo. One positive hint was provided by a glimpse at Keynote-123’s statistical analysis plan, which revealed that the study would be “considered positive if it met either [PFS or OS] primary endpoint”.
Nothing regarding OS has yet been revealed from Opdivo’s supporting Checkmate-274 trial, but the drug might already have become sufficiently established here to scupper a rival study with an observational control.
Cross-trial comparisons in adjuvant treatment of bladder cancer
Opdivo | Keytruda | Tecentriq | Imfinzi | |
|---|---|---|---|---|
| Checkmate-274 | Keynote-123 | Imvigor-010 | Niagara | |
| DFS | 20.8mth vs 10.8mth | 29.0mth vs 14.0mth | 19.4mth vs 16.6mth | Ends Jun 2025 (also includes a neoadjuvant, chemo-combo stage) |
| HR=0.70, p=0.0008 | HR=0.69, p=0.0013 | HR=0.89, p=0.24 | ||
| OS | Not disclosed | 50.9mth vs 55.8mth | NR vs NR | |
| HR=0.98, p=0.88 | HR=0.85 | |||
| Status | US approved Aug 2021 | Analysis ongoing | Trial failed |
Source: prescribing information, The Lancet & ASCO.
This is an updated version of a story published earlier.
1467













