
ASCO-GI – some shine comes off Astra’s Emerald
The first-line liver cancer Emerald-1 trial is positive, but only up to a point.
The first-line liver cancer Emerald-1 trial is positive, but only up to a point.

Revelation of survival data from AstraZeneca’s Emerald-1 trial in first-line liver cancer has confirmed the suspicion sceptics had had when the study was toplined positively last November: adding Imfinzi alone to TACE doesn’t improve PFS.
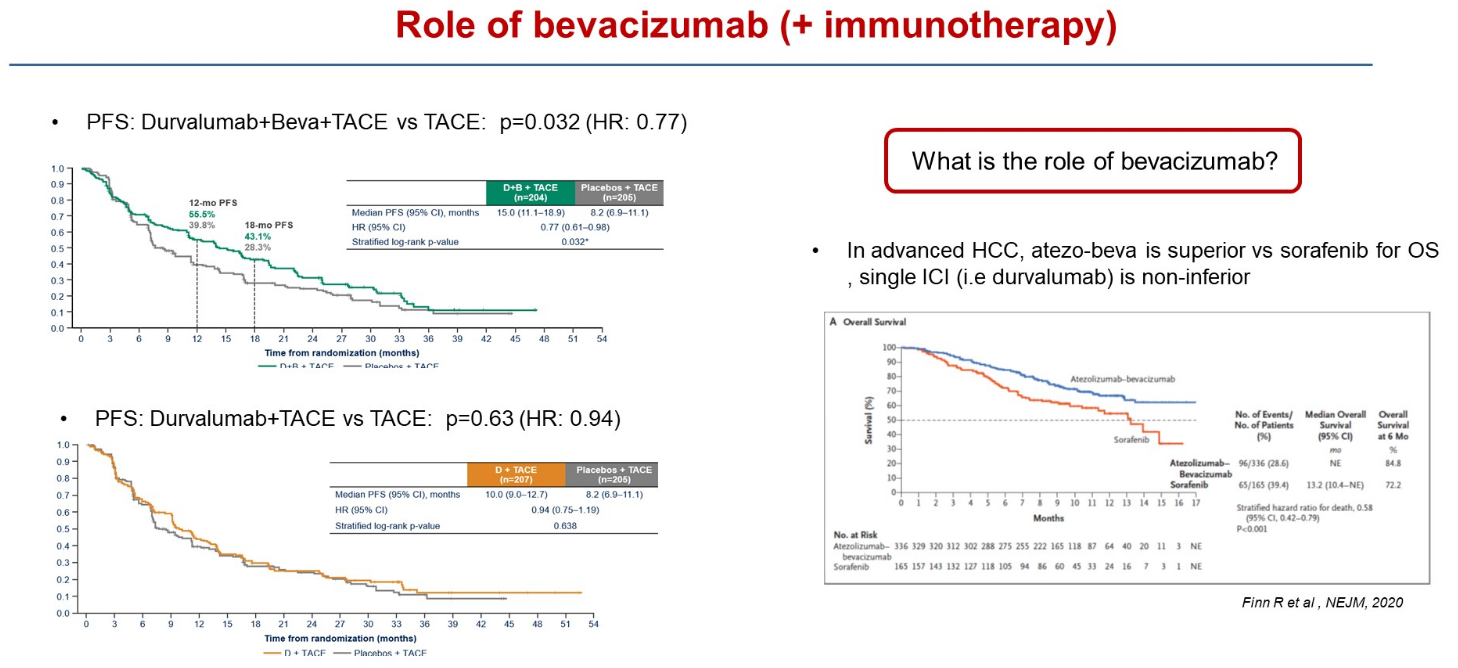
Emerald-1 concerns liver cancer patients eligible for TACE (transarterial chemoembolisation), and at ASCO’s Gastrointestinal Cancers Symposium today the claim was made that this was the first trial to demonstrate improved clinical outcomes for immunotherapy-based combinations with TACE in these patients. That’s true – but only if treatment also includes Roche’s Avastin.
This is a vital consideration, since Avastin makes up one half of a first-line liver cancer standard, as an approved combination with Tecentriq, backed by the Imbrave-150 trial. Astra’s own Imfinzi was later also approved in first-line liver cancer, as part of a combo with the anti-CTLA-4 MAb Imjudo.
In Emerald-1 there were two active cohorts: Imfinzi plus TACE with Avastin, and Imfinzi plus TACE without Avastin. Only the first has resulted in a statistically significant PFS benefit versus TACE, the primary endpoint, ASCO-GI heard; Imfinzi plus TACE did beat TACE numerically, but by less than two months.
University of Barcelona’s Professor Josep Llovet, discussant of the Emerald-1 data at ASCO-GI, specifically raised the question of Avastin, a drug he said played an important role in immunotherapy combinations in liver cancer.
Summary of data from Emerald-1
Imfinzi + Avastin + TACE | Imfinzi + TACE | TACE | |
|---|---|---|---|
| Median PFS (mth) | 15.0 | 10.0 | 8.2 |
| PFS stats vs TACE | HR=0.77, p=0.032 | HR=0.94, p=0.638 | – |
| ORR | 43.6% | 41.0% | 29.6% |
| Grade 3/4 TRAEs | 32.5% | 15.1% | 13.5% |
| Discontinuation due to TRAE | 8.4% | 4.3% | 3.5% |
Source: ASCO.
Interestingly, at least one secondary endpoint – overall response rate – does favour both active cohorts, but this hasn’t translated into a similar benefit on PFS. The key secondary of overall survival wasn't statistically significant at interim analysis, with follow-up continuing to final analysis, at which point the Avastin-containing triplet will need to clear a boundary of p=0.01.
The full data will determine which cohort regulators might decide to approve, though it’s unclear whether a PFS benefit alone will be sufficient. When Emerald-1 was toplined Astra said it would share the data with agencies, but notably it hasn’t revealed whether a formal filing has in fact been made or accepted.
Two-horse race
First-line liver cancer is effectively a two-horse race, since Keytruda and Opdivo both failed here. Emerald-1 is important for Astra because the company’s challenge against Roche in this setting has been somewhat cautious: overall survival data in the Himalaya trial supporting Imfinzi plus Imjudo’s US approval came up short of Imbrave-150 on a cross-trial basis.
Meanwhile, cross-trial comparisons of Emerald-1 versus Imbrave-150 or Himalaya are complicated by the fact that the latter two concern a different first-line setting, not being run in patients eligible for TACE. Indeed, around half of the patients in Imbrave-150 had already received TACE, so Emerald-1 can be seen as an earlier treatment niche.
A success for Astra in Emerald-1 could result in Imfinzi being given to first-line liver cancer patients before they become eligible for the Imbrave-150 regimen. However, the result at present appears primarily to underscore the importance of Avastin, which might simply play to the advantage of Roche’s currently approved front-line combo.
This is an updated version of a story published earlier.
1253













