
AACR 2024 – a first look at Astra’s ATM inhibitor
One of the first human datasets concerning an ATM inhibitor shows glioblastoma’s intractability.
One of the first human datasets concerning an ATM inhibitor shows glioblastoma’s intractability.

The extremely poor prognosis of brain cancer might account for the fact that a study of radiation therapy combined with AstraZeneca’s ATM inhibitor AZD1390, which yielded only the slightest glimmers of activity, was described at AACR yesterday as showing encouraging preliminary efficacy.
The dataset, from a multi-cohort, uncontrolled phase 1 trial now focusing purely on glioblastoma, is one of the first to report the efficacy of an ATM inhibitor in human trials. It’s notable that the only other western player active here, Merck KGaA, advanced clinical development of its ATM inhibitor lartesertib on data that appeared to be even less supportive.
ATM is a sensor of DNA damage, and its inhibition has been hypothesised to increase the efficacy of radiotherapy by preventing post-radiation DNA damage repair. Merck and Astra have each had a couple of attempts here: in 2019 the German group discontinued M3541 before turning to lartesertib, while Astra dropped AZD0156 to try its luck with AZD1390.
Radiation combo
Astra’s phase 1 trial of AZD1390 plus radiotherapy started with three cohorts: pretreated glioblastoma, solid tumours and front-line glioblastoma. But the second was terminated owing to slow recruitment, while the third is insufficiently mature for efficacy analysis; yesterday’s AACR presentation thus focused on 75 patients with recurrent glioblastoma.
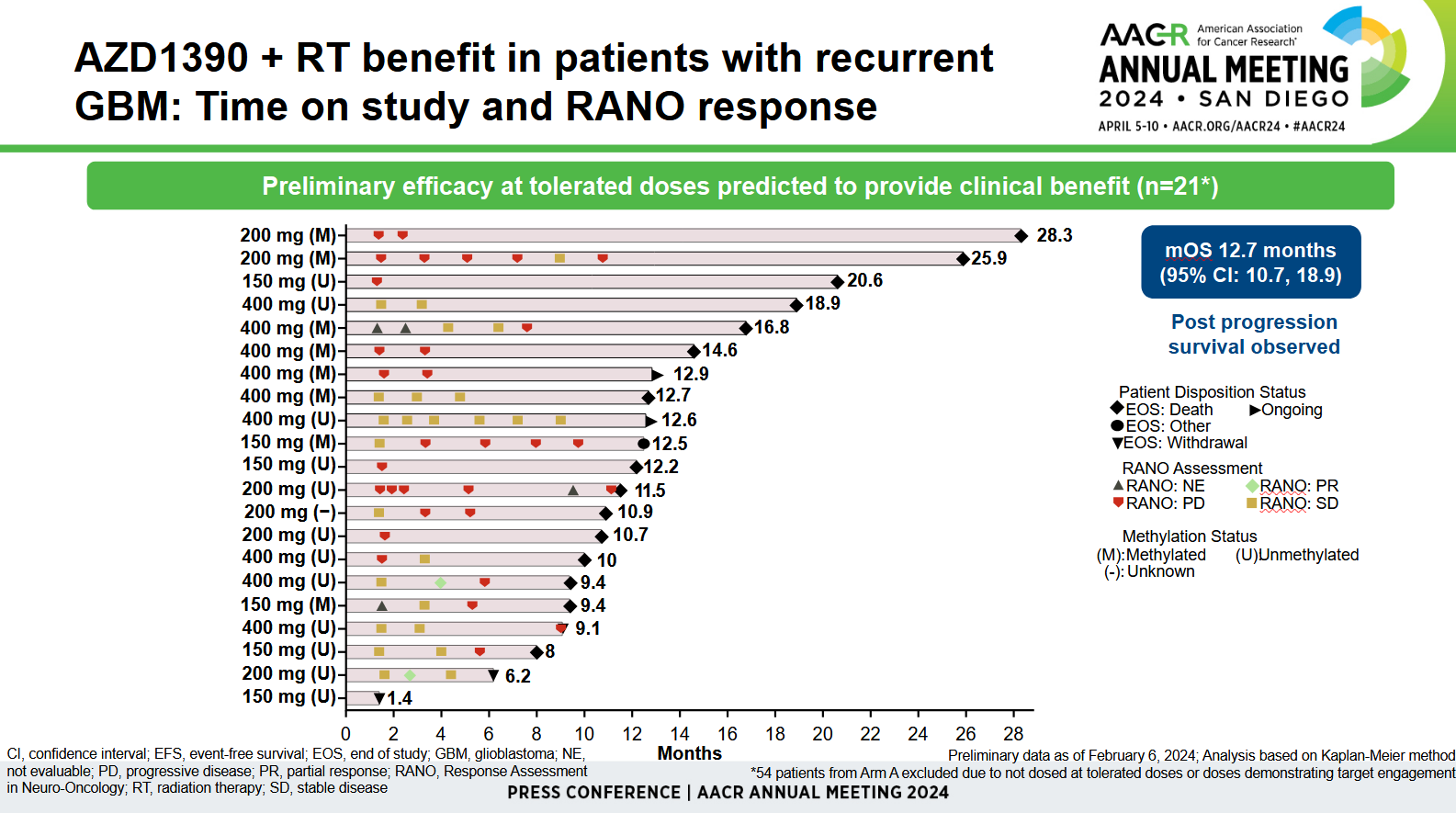
The number backing investigators’ optimism here was median overall survival of 12.7 months. This was described as promising by Memorial Sloan Kettering’s Dr Jonathan Yang, who said glioblastoma patients normally died within two years of first diagnosis, and this was a “heavily pretreated” population.
However, the AACR data concerned only a 21-patient subset of the 75-strong cohort. The remaining 54 patients were excluded because they received doses that either didn’t demonstrate target engagement, or weren’t tolerated. Moreover, across the 75-patient cohort there were only two partial responses, according to neuro-oncology criteria.
Yang said radiographic imaging could be imperfect, being confounded by pseudoprogression for instance, so the survival figure was more important. That said, the two partial responses occurred at 200mg and 400mg, which might be at the high end of tolerability; the maximum tolerated AZD1390 dose was found to be 400mg in cohort A and 300mg in cohort C.
Ultimately, no matter how scientifically plausible the ATM mechanism seems, whether anything is happening here will only be demonstrated by a controlled study, perhaps comparing AZD1390 plus radiation against radiation with or without Temodar, the mainstay of glioblastoma therapy.
It’s not clear how AZD1390 might improve on the now discontinued AZD0156, which showed two partial responses when combined with Lynparza in 46 patients with various cancers, but was said to have treatment-limiting haematologic toxicity. Meanwhile, Merck KGaA’s M3541 plus radiation yielded three responses in 15 patients, but was discontinued owing to suboptimal pharmacokinetics and lack of a dose–response relationship.
Merck’s current effort in ATM focuses on lartesertib, which entered the phase 1 DDRiver Solid Tumors 320 trial, having shown nothing beyond target engagement, relative safety and the establishing of 300mg daily as the maximum tolerated dose in the earlier DDRiver Solid Tumors 410 study. The remaining work in ATM inhibition is at Chinese companies.
Biopharma industry ATM inhibitors
| Project | Company | Status |
|---|---|---|
| Lartesertib (M4076) | Merck KGaA | Ph1 DDRiver Solid Tumors 410 trial, completed Mar 2023 |
| Ph1 DDRiver Solid Tumors 320 trial, ATRi/PD-L1 MAb combo | ||
| AZD1390 | AstraZeneca | Ph1 radiotherapy combo in brain tumours |
| SYH2051 | CSPC Pharmaceutical | Ph1 monotherapy or radiotherapy combo in head & neck cancer |
| WSD0628 | Wayshine Biopharm | Ph1 IST, radiotherapy combo in brain tumours |
| ZN-B-2262 | Suzhou Zanrong Pharmaceutical | Ph1 in head & neck cancer (China study) |
| IMP08 | Impact Therapeutics | Preclinical |
| M3541 | Merck KGaA | Ph1 terminated 2019 (suboptimal PK profile) |
| AZD0156 | AstraZeneca | Ph1 completed 2019; discontinued after treatment-limiting haematologic toxicity |
Source: OncologyPipeline.
1489













