
Ifinatamab goes pivotal

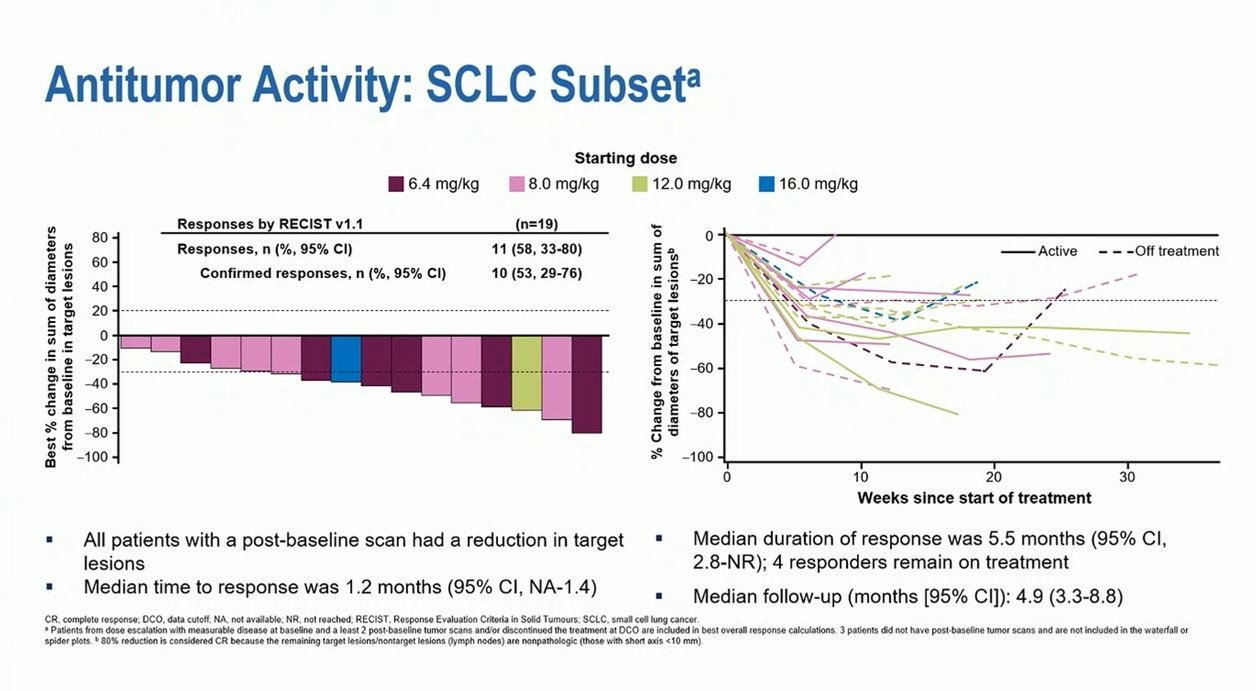
In March Daiichi Sankyo/Merck & Co will begin only the third clinical trial of ifinatamab deruxtecan, but already this will be a pivotal study. A clinicaltrials.gov entry just published reveals that this phase 3 trial, called Ideate-2, will test the anti-B7-H3 ADC against physician’s choice of topotecan, amrubicin or Zepzelca in post-platinum small-cell lung cancer, and measure ORR and overall survival as co-primary endpoints. At ESMO 2022 ifinatamab yielded data from a phase 1/2 solid tumour trial, in which 19 SCLC patients showed a 53% rate of confirmed Recist responses; the phase 2 Ideate-1 trial specifically in SCLC won’t read out until this year. As for comparator expectations, Zepzelca’s label, in a broadly similar post-platinum SCLC population, cites a 35% ORR, or 45% in patients with a chemo-free interval over 90 days. Ifinatamab’s fast progress will be noted by competitors in the B7-H3 space, which recently saw GSK license Hansoh Pharma’s HS-20093, and AbbVie present phase 1 data for ABBV-155 at ASCO. MacroGenics’ vobramitamab duocarmazine is due to yield phase 2 prostate cancer data this year, while Y-Mabs’ iodine-131 labelled omburtamab received US refuse-to-file and complete response letters, and no longer appears in the group’s pipeline.
SCLC data from ifinatamab deruxtecan's phase 1/2 basket study
1851













