
World Lung 2023 – targeted therapy to the rescue in small-cell lung
Adding a kinase inhibitor to PD-L1 plus chemo could make a real difference in first-line SCLC, a little-appreciated Chinese trial suggests.
Adding a kinase inhibitor to PD-L1 plus chemo could make a real difference in first-line SCLC, a little-appreciated Chinese trial suggests.

Though the past four years have seen not one but two anti-PD-(L)1 drugs approved for front-line small-cell lung cancer, the benefit they bring is pretty marginal. Now a Chinese multicentre study has suggested that adding a tyrosine kinase inhibitor into the mix might significantly increase patients’ survival.
The data concern Sino Biopharm’s little-known anti-PD-L1 MAb benmelstobart, which when combined with the locally approved multikinase drug anlotinib and chemo saw first-line SCLC patients live on average 19.3 months, the World Lung conference has just heard. Not only is this seven months longer than chemo alone, it’s over six months more than either Tecentriq or Imfinzi managed in their registrational studies.
It should be stressed that such a claim can only be made on a cross-trial basis, which carries significant caveats as to trial design and baseline characteristics. In addition, some in the west might question the reliability of data generated solely in China.
That said, Sino’s study, Eter-701, comprised 72 hospitals, and used quadruple blinding in a formal phase 3 design. Even if its results will be questioned, it does at least point to the future potential of adding targeted therapy on top of anti-PD-(L)1 chemo-combos.
New lead indication?
Sino, through its Chia Tai Tianqing subsidiary, is developing benmelstobart (until recently coded TQB2450 or CBT-502) for several indications, with phase 3 studies including NSCLC, head and neck triple-negative breast and cervical cancers.
On the strength of Eter-701, however, first-line SCLC might have become its most immediate potential use. Presenting the data at World Lung today Dr Ying Cheng, from Jilin Cancer Hospital, called the triplet’s overall survival benefit “historically long”. Eter-701 also included an anlotinib plus chemo doublet cohort, but data from this were not presented.
The triplet’s median OS came in at 19.3 months, versus 11.9 months for chemo alone. This cut risk of death by 39% across the entirety of the trial, yielding a highly statistically significant p value of 0.0002. Moderating this morning's World Lung press briefing, Montefiore-Einstein Cancer Center's Dr Brendon Stiles called this kind of survival curve separation "quite remarkable".
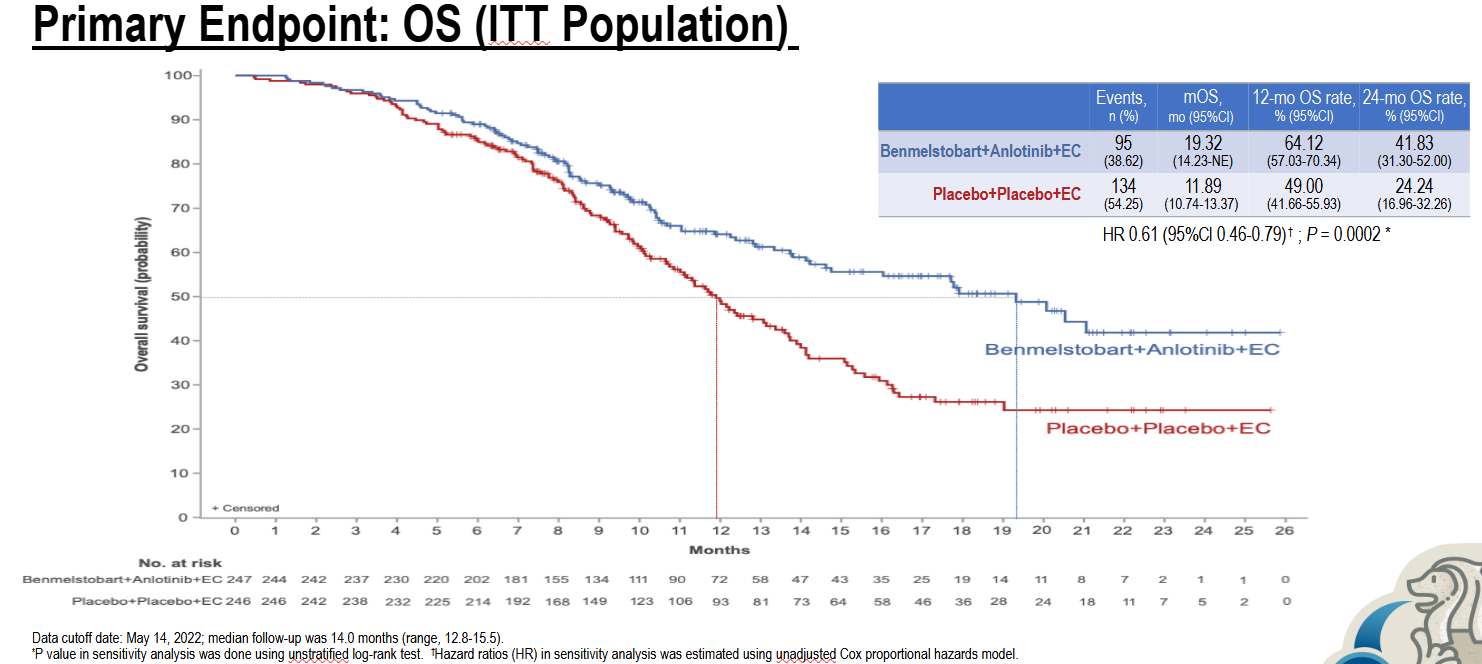
In comparison, Roche’s Impower-133 showed Tecentriq plus chemo to cut risk of death by 30%, while in AstraZeneca’s Caspian Imfinzi plus chemo yielded a 0.73 hazard ratio – both enough to secure US approvals. However, both chemo combos beat chemo alone by less than three months, suggesting that their data lacked strong clinical meaningfulness, despite being clearly statistically significant.
Other PD-(L)1 drugs have fared less well. Bristol Myers Squibb’s Checkmate-451 study, in the front-line maintenance setting, did not show a benefit either for Opdivo monotherapy or for Opdivo plus Yervoy. Merck & Co failed in Keynote-604 with Keytruda plus chemo, which at median showed only a 1.1-month benefit over an underperforming chemo cohort.
Selected phase 3 first-line SCLC trials
| Drug | Company | Study | Setting | mOS result |
|---|---|---|---|---|
| Benmelstobart | Chia Tai Tianqing (Sino Bio) | Eter-701 | + anlotinib + chemo, vs chemo | 19.3mth vs 11.9mth (HR=0.61, p=0.0002) |
| Imfinzi | Astrazeneca | Caspian | + chemo, vs chemo | 13.0mth vs 10.3mth (HR=0.73, p=0.005)* |
| Tecentriq | Roche | Impower-133 | + chemo, vs chemo | 12.3mth vs 10.3mth (HR=0.70, p=0.007) |
| Keytruda | Merck & Co | Keynote-604 | + chemo, vs chemo | 10.8mth vs 9.7mth (HR=0.80, failed) |
| Opdivo | Bristol-Myers Squibb | Checkmate-451** | Monotherapy, vs placebo | 10.4mth vs 9.6mth (HR=0.84, failed)^ |
Notes: *Imjudo-containing triplet failed; **first-line maintenance setting; ^Yervoy-containing doublet also failed. Source: IASLC & product labels.
An obvious question, however, is whether adding a triplet might add unacceptable toxicity, especially as tyrosine kinase inhibition is known to be associated with significant adverse events.
In Eter-701 grade 3 or higher serious adverse events rose from 34% for chemo to 47% for the triplet, though liver enzyme elevations, for instance, did not appear materially different. Cheng called the triplet’s safety profile “tolerable”.
So should pharma now test PD-(L)1 plus chemo additionally with a tyrosine kinase inhibitor in first-line SCLC? Possible targeted agents include Eisai’s Lenvima or Pfizer’s Inlyta, both of which are approved in PD-1 combos in kidney cancer; Merck is testing Keytruda with chemo and numerous other agents, including Lenvima, in the first-line SCLC trial Keynote-B99, though this is uncontrolled and phase 2.
Little other work appears to be ongoing at present, with apparently the only other phase 3 study being an academic-sponsored trial of Jiangsu Hengrui’s camrelizumab plus apatinib and chemo. The World Lung data might focus several minds.
897













