Tightening the Bolt on a questionable strategy

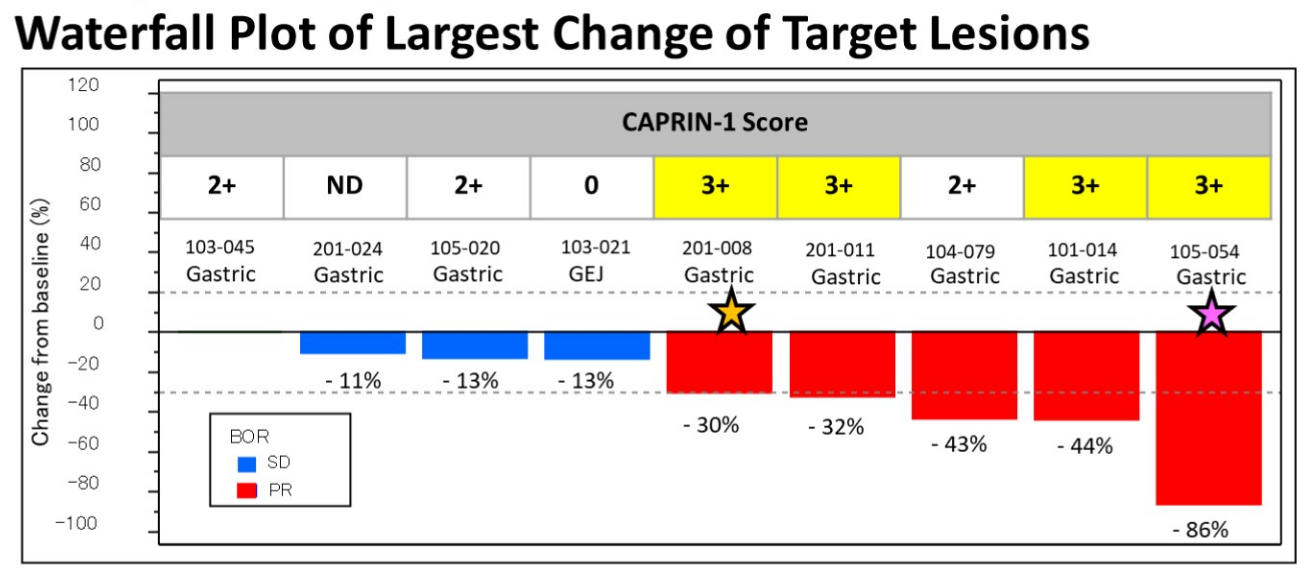
Bolt Therapeutics' failure with a souped-up antibody hasn't stopped the company from persevering with this approach – against a different target. Bolt overhauled its C-suite last year in the wake of the failure of trastuzumab imbotolimod, an anti-HER2 MAb linked to a TLR7/8 agonist, and it has now unveiled plans to take an anti-Caprin-1 MAb and develop it as a similar immune-stimulating antibody conjugate (ISAC). The aim of such ISACs, of which trastuzumab imbotolimod was an example, is to activate the immune system in addition to hitting a tumour-specific target. Though none other than Pfizer is pursuing a similar plan with its preclinical anti-PD-L1/TLR7 agonist PF-08046037, these molecules have had limited success, and Silverback Therapeutics threw in the towel with TLR8 agonist ISACs. Bolt's latest play is the result of a 2019 tie-up to develop a Toray MAb as an ISAC, and that MAb has just been revealed to be TRK-950. OncologyPipeline shows TRK-950 to be the industry's only project to target Caprin-1; data presented at ASCO-GU in 2023 showed a 56% ORR in nine evaluable gastric cancer patients. Bolt hasn't yet specified whether its ISAC version of TRK-950 will use a TLR7/8 agonist payload.
TRK-950 in phase 1
1239













