
A second attempt at heating up Anktiva

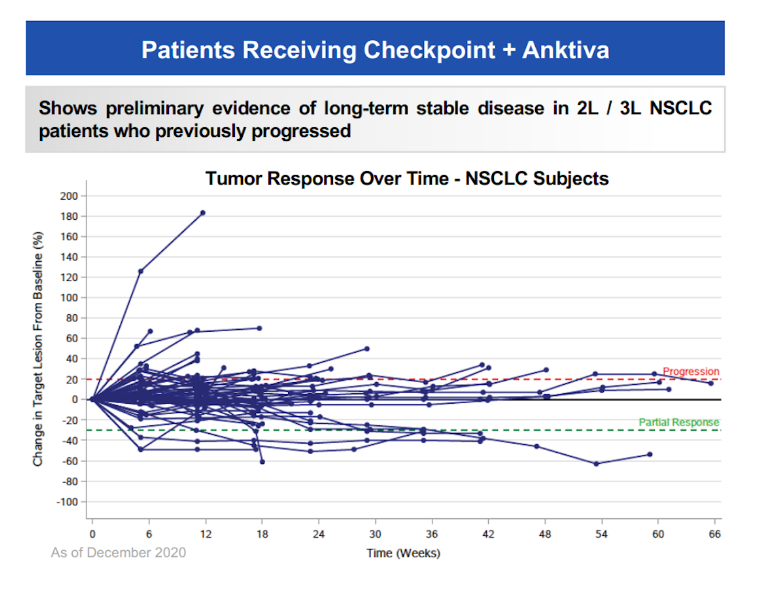
ImmunityBio is making hay while the sun shines. Fresh from Anktiva’s US approval the group has claimed an “almost double” overall survival result in Anktiva’s Quilt 3.055 trial in second/third-line NSCLC. The big caveat is that Quilt 3.055 is an uncontrolled phase 2 study, so ImmunityBio’s claim of a “significant prolongation of OS” is based solely on a comparison against historical data for established chemotherapy, and without a direct comparator any imbalances in patients’ baseline characteristics can’t be accounted for. Since all patients had progressed after PD-(L)1 therapy, and received Anktiva, an IL-15 agonist, plus anti-PD-(L)1, the implication is that “cold” tumours are being reactivated and becoming immunogenic again – a dream scenario for cytokine therapies. Still, Quilt 3.055 is a relatively small basket trial, and only about 50% of its 147 enrollees had NSCLC. As for what typical median OS for post-PD-(L)1 patients might be, ImmunityBio cites 7-9 months, but the recent Tropion-Lung01 trial of datopotamab deruxtecan yielded 11 months in its chemo control arm. Earlier data from Quilt 3.055 showed only a handful of patients responding. ImmunityBio wants to discuss the OS numbers with regulators, but a call for a controlled trial versus chemo seems the likeliest outcome.
Response data from Quilt 3.055
1692













