
ESMO 2024 – Bristol bucks the degrader trend

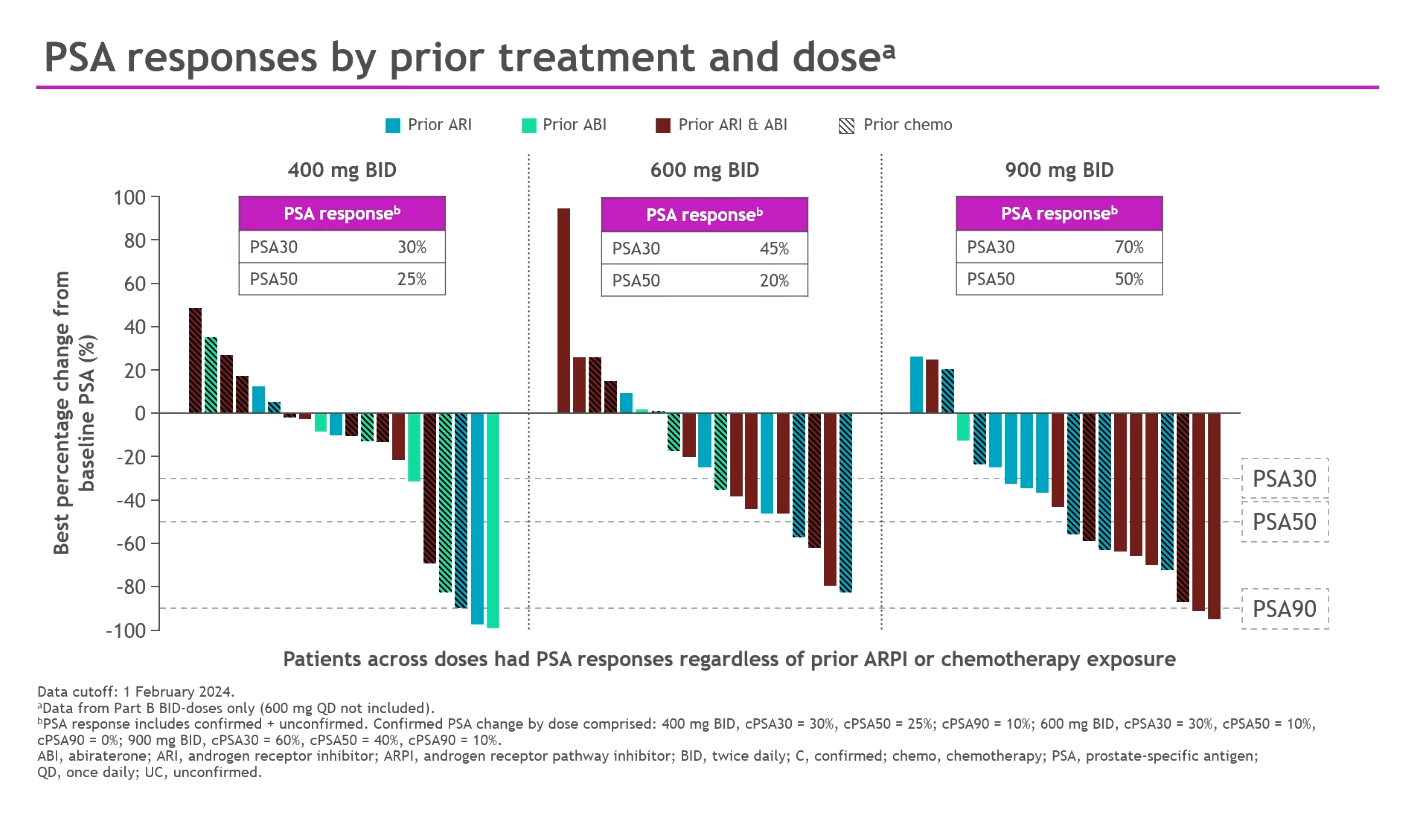
While targeted degradation promised to be a major theme of ESMO the data from Prelude, C4 Therapeutics and Astellas were lacklustre. One company bucking this trend is Bristol Myers Squibb, which revealed plans to take its AR “degrader and antagonist” BMS-986365 straight into phase 3 in metastatic castration-resistant prostate cancer in 2025, having only run one phase 1 study, whose results were updated on Monday. The data supported Bristol’s enthusiasm: 400-900mg twice-daily in dose expansion produced PSA30 and PSA50 responses of 30-70% and 25-50% respectively – despite all these mCRPC patients having failed on Zytiga or Xtandi, and 46% having failed both. One cautious note was a dose-limiting toxicity, prolonged QTc, at 900mg; Bristol didn’t reveal the phase 3 dose. The company says BMS-986365 comprises an AR-binding moiety that inhibits competitively, and a cereblon-binding moiety that facilitates targeted ubiquination and proteasome-mediated AR degradation; it could overcome Xtandi/Zytiga resistance regardless of AR mutation. Arvinas recently licensed its AR degrader ARV-766 to Novartis, but bavdegalutamide, an earlier AR degrader Arvinas had hoped to put into phase 3 this year, has been discontinued. After Opdualag and BMS-986012, BMS-986365 is the third Bristol project going pivotal on the back of ESMO data.
2809













