ESMO 2024 – a role for immunotherapy in ovarian cancer
If the therapeutic window remains open, OncoC4/BioNTech’s gotistobart could hold promise.
If the therapeutic window remains open, OncoC4/BioNTech’s gotistobart could hold promise.

Gotistobart, an improved anti-CTLA-4 MAb that BioNTech licensed from OncoC4 for $200m last year, might help immunotherapy break into ovarian cancer, an ESMO presentation on Sunday suggested. That’s despite gotistobart doses in the phase 2 study in question having been reduced because of a toxicity signal.
Though gotistobart is claimed to have a smart design, using a pH-sensitive molecule to avoid autoimmunity-related side effects, it still causes toxicity and patient discontinuations, so a careful path will have to be trodden towards a therapeutic window. But its activity in ovarian cancer is notable given this is a tumour that's thought of as immunologically “cold”.
This might explain why no anti-PD-(L)1 drugs are approved specifically for ovarian cancer, though there were some responses in a study backing Keytruda’s tumour-agnostic approval for dMMR/MSI-high cancers. The Keynote-100 trial, for instance, saw the Merck drug yielding “modest” activity in ovarian cancer, with ORRs of 7-18% depending on PD-L1 expression.
Promising responses
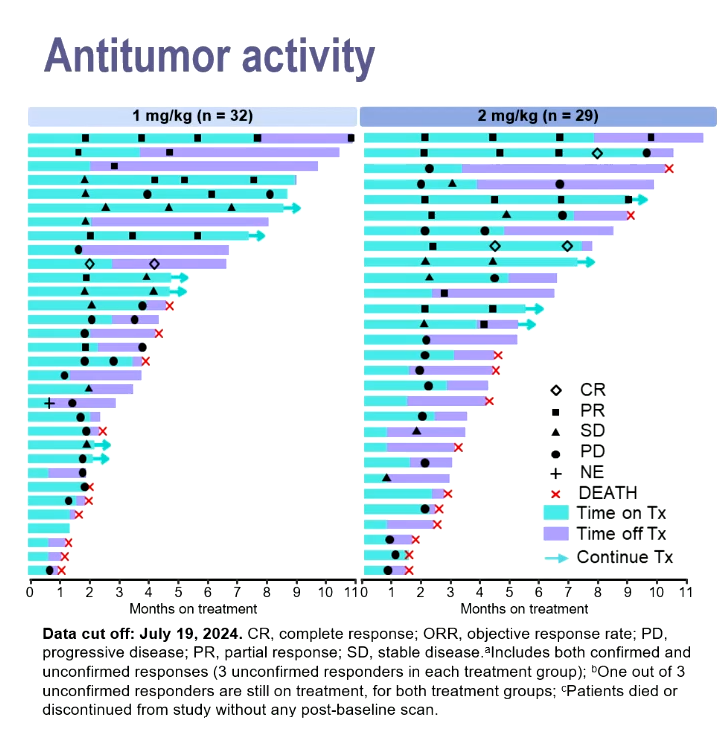
Against that precedent the data gotistobart just yielded in the phase 2 Preserve-004 study, where 1mg/kg or 2mg/kg doses were combined with Keytruda, look promising.
Dr Joyce Barlin, of Women’s Cancer Care Associates, New York, reported unconfirmed response rates of 25% with the former dose and 28% with the latter, and said these included three complete remissions among the total 61 subjects. Patients had previously received one to nine treatments, and were resistant to platinum chemo and Avastin.
A notable aspect of the swimmers plot presented was that several partial responders appeared to remain in response after coming off therapy.
Since OncoC4, a private US biotech, licensed gotistobart to BioNTech for $200m up front, the two companies have started the phase 3 Preserve-003 study in the tough setting of PD-(L)1-relapsed NSCLC.
Barlin revealed that Preserve-004 started with the aim of giving patients higher gotistobart doses, namely 3mg/kg and 6mg/kg, and in fact 21 subjects had received these. But these had to be dialled down after toxicities seen in a phase 1 basket trial, Preserve-001.
Nevertheless, the new lower dosing was still associated with a worrying level of toxicities. Serious adverse events at grade 3 or higher deemed to be drug related were seen in 55% and 72% of patients given 1mg/kg and 2mg/kg respectively; toxicity led to gotistobart discontinuation in 21% and 28% of patients.
This might seem strange in a MAb specifically designed to be safer. Gotistobart is a pH-sensitive molecule, and the aim is to prevent the degradation of it and its target. In theory, once internalised gotistobart and CTLA-4 are dissociated and recycled, and this cycling of CTLA-4 is meant to maintain Treg function and thus prevent autoimmune reactions.
Despite these claims, 46-62% of patients in Preserve-004 suffered immune-related adverse events, 24-38% at grade 3 or above. It seems that fears about the toxicity of targeting CTLA-4 – think Yervoy – aren’t about to be allayed.
2023













