
An alpha turnaround for Relay
The fortunes of RLY-2608 reverse, and a pivotal study will follow.
The fortunes of RLY-2608 reverse, and a pivotal study will follow.

After Lilly doubled down on alpha isoform-specific PI3K inhibition attention fell on Scorpion’s take on this mechanism, STX-478, due to be profiled at this week’s ESMO conference. However, on Monday it was a third PI3Kα inhibitor that seized the limelight, as a clinical update on Relay Therapeutics’ RLY-2608 prompted that biotech’s stock to surge 52%.
This appears to be quite the turnaround for a molecule that until now has underwhelmed. Though mechanistically RLY-2608 was shown to be doing precisely what it was meant to, first-in-human data showed zero confirmed responses at last year’s AACR meeting; that number has now come in at a more impressive 27% as part of a Faslodex combo.
The setting is HER2-negative, ER-positive metastatic breast cancer that’s driven by the PIK3CA mutation. PI3Kα inhibition is seen as an ideal mechanism to target this mutation, but activity at other PI3K isoforms has caused significant toxicity, and held back several projects with this pharmacology.
One exception is Novartis’s Piqray, which was approved in 2019 with a label warning of numerous toxicities, most notably severe hyperglycaemia. Another PI3Kα inhibitor, Roche’s inavolisib, faces a 27 November PDUFA date after being filed for PIK3CA-mutated breast cancer on the back of the phase 3 Inavo-120 trial.
Less toxic?
Relay claims to have dialled up alpha isoform specificity to such an extent that RLY-2608 is meaningfully less toxic than other PI3Kα inhibitors. The trial updated today, Rediscover, showed around a 45% rate of hyperglycaemia, but only 2% was at grade 3, and 30% was at grade 1 with no intervention required. There were no grade 4 or 5 treatment-related adverse events, Relay said.
As for efficacy, the key focus of Relay’s update was a combo of RLY-2608 600mg twice daily with Faslodex in 30 efficacy-evaluable patients. Among these there were eight confirmed responses and a further two that were unconfirmed. Another patient had an unconfirmed complete response, but was omitted for having had non-measurable disease at baseline.
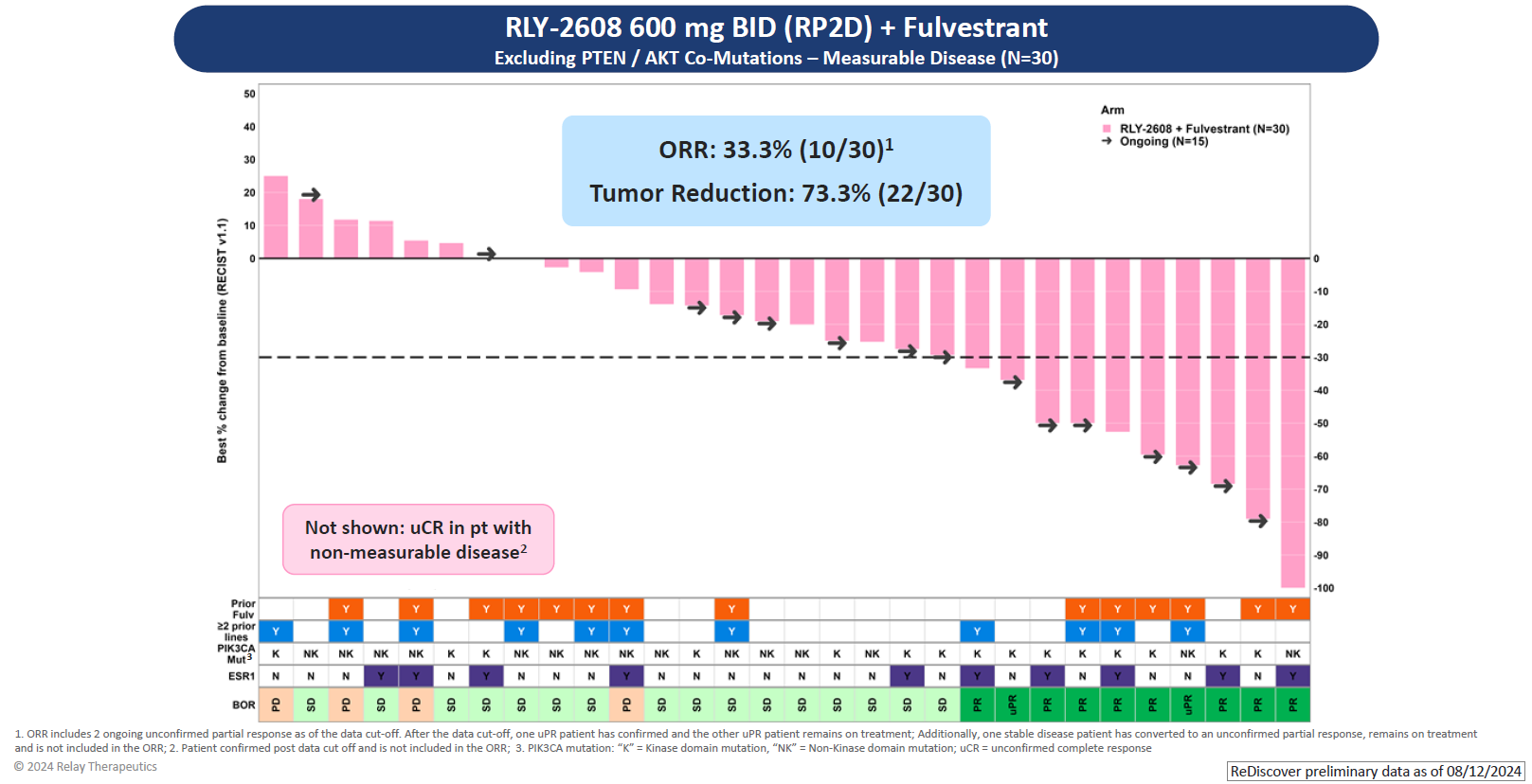
If roughly a third of patients are responding that's a turnaround for this project, compared with its AACR 2023 disappointment. At that point 16 patients were evaluable, two having received RLY-2608 monotherapy and the rest the Faslodex combo; there was just one response – unconfirmed – in a monotherapy subject.
Alpha-selective PI3K inhibition came into focus after Lilly recently discontinued LOXO-783, specifically an H1047R mutant-selective project, but talked up the prospects of improved follow-on assets. Scorpion’s STX-478 is being tested in PI3Kα H1047-mutant breast cancer, and has an ESMO late-breaker due to be presented on Sunday.
On the back of the Rediscover update Relay says it plans next year to start a pivotal study of the RLY-2608/Faslodex combo in PIK3CA-mutated breast cancer patients refractory to one line of CDK4/6 therapy. The comparator, however, won’t be Piqray but rather AstraZeneca’s AKT inhibitor Truqap, recently approved for PIK3CA/AKT1/PTEN-altered breast cancer.
Relay says its combo’s response rates and 9.2-month median PFS, also revealed today, compare favourably against the respective 26% and 5.5 months that Truqap plus Faslodex showed in the CAPItello-291 trial; Piqray plus Faslodex has yielded broadly similar numbers.
However, Roche’s inavolisib has shown median PFS of 7.1 months so, depending on the FDA’s verdict, that might end up being the drug Relay goes up against in the real world.
1384













