
World Lung 2024 – picking apart the Harmoni-2 win
Akeso’s data look impressive, but perhaps not impressive enough to sustain Summit’s valuation.
Akeso’s data look impressive, but perhaps not impressive enough to sustain Summit’s valuation.

Akeso/Summit’s ivonescimab has indeed “decisively” beaten Keytruda in the front-line lung cancer study Harmoni-2. That much is clear from late-breaking full data from the Chinese study, presented for the first time at Sunday's World Lung presidential session.
The bigger question, however, is whether Keytruda performed as expected, and here things become sketchy: in the PD-L1-high subgroup the Merck & Co drug seems to have come up short of the Keynote-024 trial. True, in Harmoni-2 all-comers ivonescimab’s win is decisive, but that’s only on PFS and ORR – two metrics that Keytruda’s separate Keynote-042 study showed to be unreliable.
Keynote-042 is a reasonable cross-trial comparator, having enrolled first-line NSCLC patients expressing PD-L1 at ≥1%, comparing monotherapy against chemo, and providing an analysis of the PD-L1 high (≥50%) subgroup. The World Conference on Lung Cancer saw presentation of similar efficacy metrics from Harmoni-2, which compared ivonescimab, a PD-1 x VEGF bispecific, against Keytruda, also in ≥1% expressers.
On Harmoni-2’s primary endpoint, PFS, ivonescimab recorded a resounding victory, cutting risk of progression or death by 49% (p<0.0001); responses were also higher, with ORR coming in at 50% for the Akeso/Summit drug versus 39% for Keytruda.
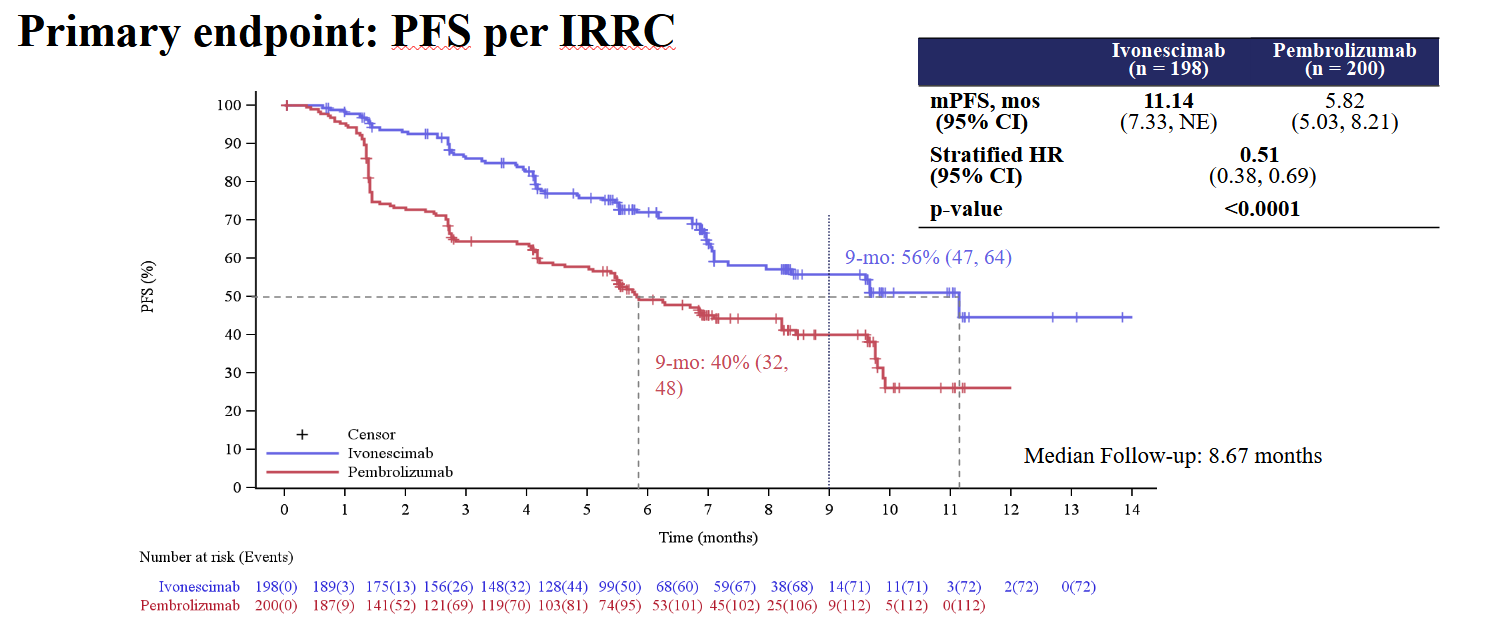
On a straight cross-trial comparison against Keynote-042 these numbers look sound, as in Merck’s trial Keytruda scored 5.4 months of mPFS and an ORR of 27%. But the problem with Keynote-042 is that Keytruda decisively failed to beat chemo in terms of PFS or ORR; it was only overall survival that showed a benefit, which backed the Merck drug’s approval.
Such a result urges caution, as it suggests that surrogate endpoints, however impressive they seem, don’t paint a true picture, and only OS data will show the value of ivonescimab. World Lung revealed nothing about OS, with the presentation saying only that this would be reported in future.
The good news was that safety appeared manageable, with the only notable treatment-related adverse event differences for ivonescimab versus Keytruda in Harmoni-2 being slight rises in low-grade liver enzyme elevation and an increase in serious TRAEs from 32% to 41%.
But further concern comes from a cross-trial comparison against Keynote-024, an early trial of Keytruda in first-line NSCLC expressing PD-L1 at ≥50%. On this basis Keytruda might have underperformed in Harmoni-2; ivonescimab’s 11.2 months’ mPFS looks barely different from the 10.3 months Keytruda scored in this patient population in Keynote-024.
True, Keynote-024 is now a rather old trial, having backed a Keytruda approval way back in 2016. Treatment paradigms have changed, so perhaps the comparison is unfair, but nevertheless it serves as another cautionary note.
Relevant cross-trial comparisons
| Ivonescimab | Keytruda | Chemo | Stats | |
|---|---|---|---|---|
| PFS in PD-L1 ≥1% | ||||
| Harmoni-2 | 11.1mth | 5.8mth | – | HR=0.51 (p<0.0001) |
| Keynote-042 | – | 5.4mth | 6.5mth | HR=1.07 |
| PFS in PD-L1 ≥50% | ||||
| Harmoni-2* | 11.2mth | 8.2mth | – | HR=0.46 |
| Keynote-042 | – | 6.9mth | 6.4mth | HR=0.82 |
| Keynote-024 | – | 10.3mth | 6.0mth | HR-0.50 (p<0.001) |
Note: *precise medians for PFS in PD-L1 ≥50% expressers were not provided, so the numbers were read off the Kaplan-Meier curves. Source: IASLC & Keytruda prescribing information.
A broader question is not whether ivonescimab is better than Keytruda, but whether it’s better than Keytruda plus Avastin. PD-1 and VEGF inhibitors are already used in NSCLC, but Summit has claimed that the bispecific ivonescimab could have improved efficacy over using two separate agents.
So far there’s no easy way to prove this, as there appears to be no obvious cross-trial comparison of such a drug combo, without chemo, in PD-L1 ≥1% first-line NSCLC. One comparison is in post-EGFR patients, where the separate Harmoni-A trial showed ivonescimab plus chemo to be no better on mPFS than Innovent’s Tyvyt/bevacizumab/chemo triplet in the Chinese registrational Orient-31 study.
This might all seem like nitpicking, but Summit paid Akeso $500m up front for ivonescimab rights. The Harmoni-2 data sent Summit's stock up 56%, and as group sits on a $14bn valuation; how watertight Harmoni-2 really is, and whether these Chinese data can be reproduced in a US registrational trial, remain key considerations for investors.
This story has been updated.
4630













