More Cartitude-4 fears allayed

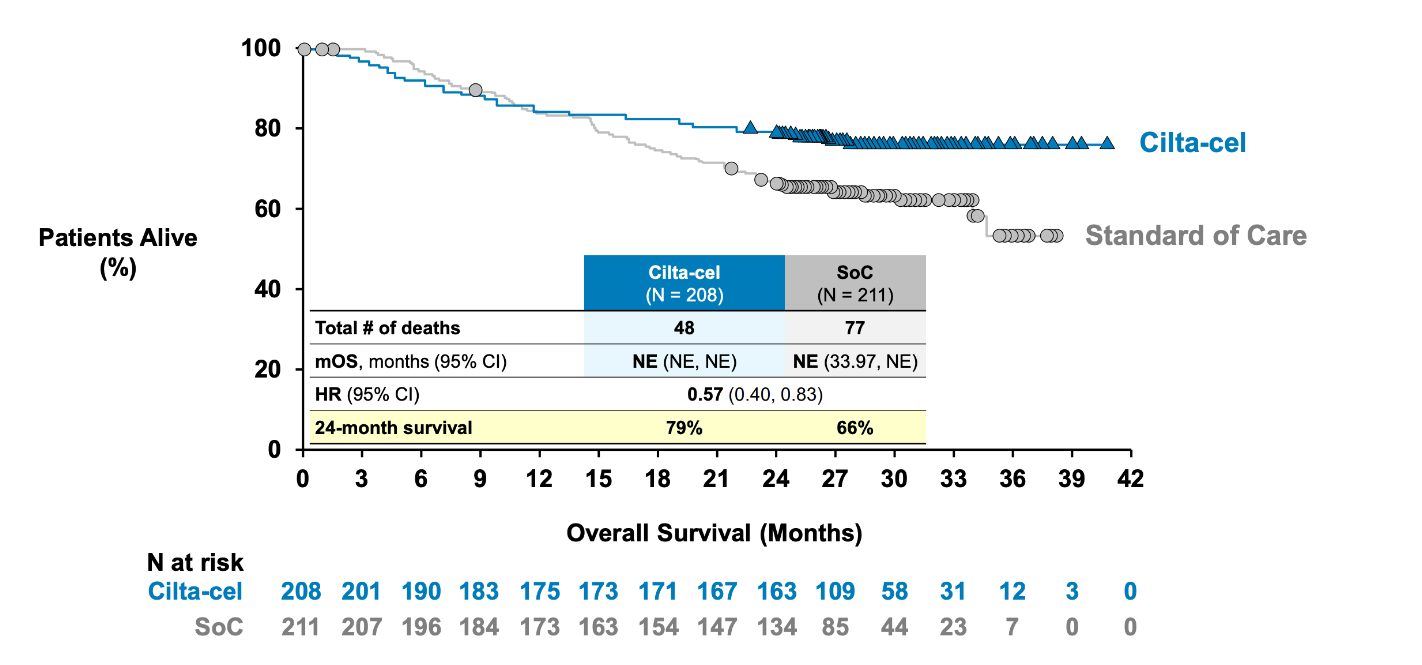
Since Carvykti secured US second-line multiple myeloma approval in April Johnson & Johnson/Legend’s claim today that the supporting Cartitude-4 trial has yielded “statistically significant and clinically meaningful improvement in overall survival” at interim analysis might pass unnoticed. But it could give doctors more confidence to prescribe the Car-T therapy in early lines, given the doubts about OS at a pre-approval US advisory committee meeting in March. In particular the fact that, at 34% maturity, there were more Carvykti deaths versus control over the first 10 months in Cartitude-4 caused alarm; this related to a November 2022 data cutoff, and is reflected in current Carvykti prescribing information showing OS curves crossing over and suggesting no benefit. An updated OS cut, as at December 2023, was presented at the adcom, and this still showed the curves crossing over, but now with survival “tails” developing, and a 43% reduction in risk of death favouring Carvykti. The early Carvykti mortality was put down to patients dying before being able to receive the therapy, and this appeared to assuage panellists. The latest J&J revelation comes as the icing on the cake, and full numbers and survival curves should be eagerly awaited.
Early OS analysis in Cartitude-4
949













