
The predictable failure of trilaciclib
The CDK4/6 inhibitor flunks its phase 3 study – a result that should have been foreseen five years ago.
The CDK4/6 inhibitor flunks its phase 3 study – a result that should have been foreseen five years ago.

G1 Therapeutics’ dream for trilaciclib in breast cancer started five years ago with an unexpected phase 2 success, and today it’s ended with a phase 3 failure the company has also deemed fit to call “unexpected”.
Trilaciclib is a CDK4/6 inhibitor whose development is geared not towards cancer treatment but at making chemo more tolerable, specifically by reducing chemotherapy-induced myelosuppression. That idea took a major knock today as the company admitted that in its phase 3 Preserve-2 study, in triple-negative breast cancer, trilaciclib plus chemo failed to beat chemo alone on overall survival.
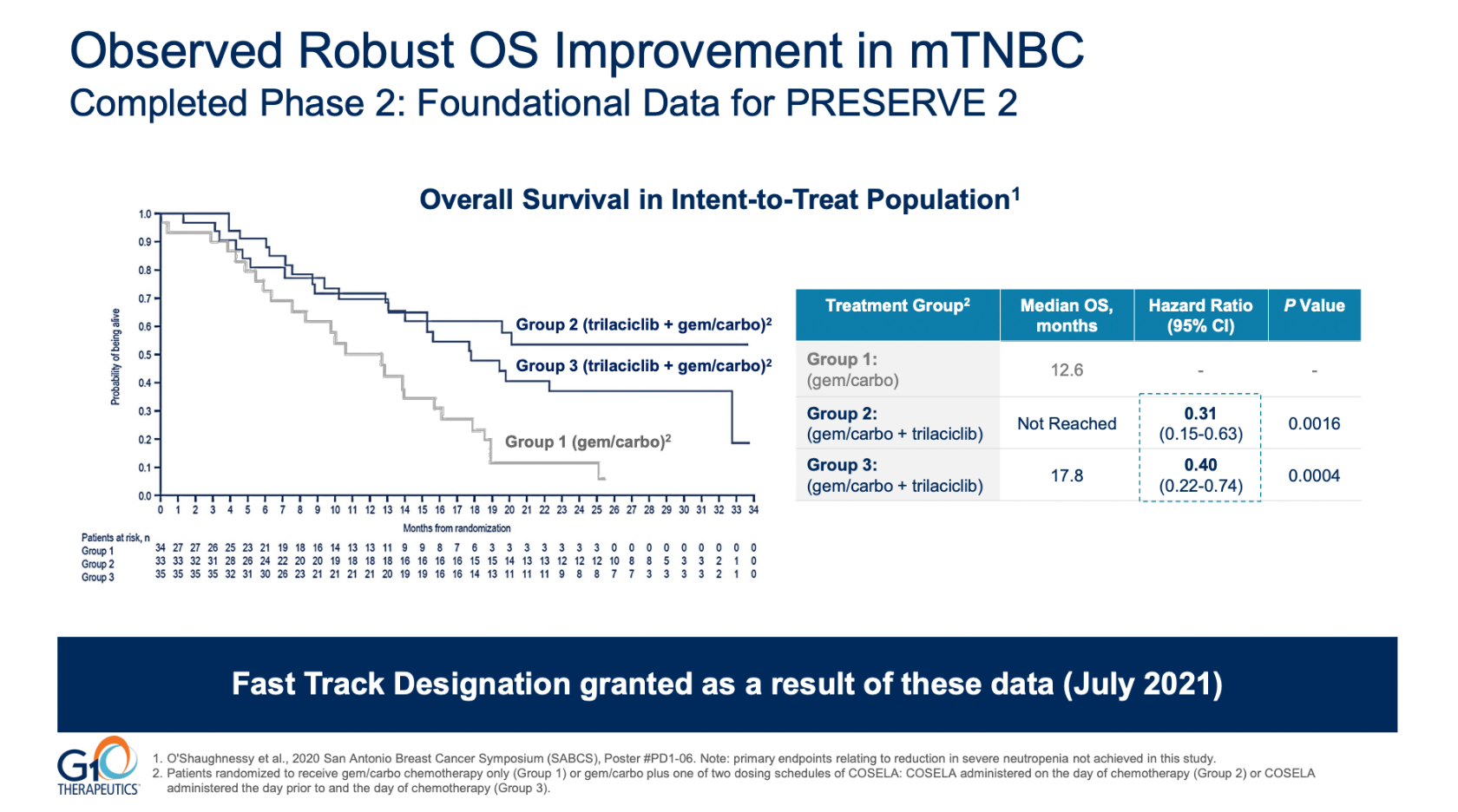
So unequivocal was the failure that medians numerically favoured the control group, though G1 might salvage some pride by citing a hazard ratio of 0.91, and through its claim that numerically OS favoured trilaciclib in PD-L1 positive and negative patients alike. These are likely chance findings.
Unexpected?
G1, which lost 20% this morning, called the failure unexpected, but in fact the study that led to Preserve-2 being run, a phase 2 trial, was itself said to have yielded an unexpectedly positive outcome – by none other than its own presenting author.
That phase 2 test failed its primary endpoints but appeared to succeed on OS – a result that was called into question by Dr Joyce O’Shaughnessy of Baylor University Medical Center, who presented those data at a late-breaker at ESMO in 2019.
Specifically, O’Shaughnessy pointed to the exploratory nature of that OS analysis, and to the fact that trilaciclib failed in that trial to reduce neutropenia or prolong patients’ PFS. The failure to reduce neutropenia in particular called into question the purported OS benefit, given trilaciclib’s proposed anti-myelosuppressive mechanism of action.
Exploratory analysis of trilaciclib's failed phase 2 trial
Trilaciclib earlier failed the phase 3 Preserve-1 study in colorectal cancer, but it is approved, as Cosela, for use before small-cell lung cancer patients are given platinum, etoposide or topotecan, on the basis of three mid-stage studies that tested not survival but neutropenia reduction.
G1 today said it would focus on that SCLC opportunity as a way to becoming a profitable business by the second half of next year. In the meantime, its experience with trilaciclib in triple-negative breast cancer should serve as a reminder that an exploratory hit in phase 2 frequently doesn’t translate into a positive outcome in a large, prospective phase 3 study.
Those investors who did bet on Preserve-2 might reflect on the fact that its failure appears to have been anything but unexpected.
1461













