
ASCO 2024 – two more Echelon-3 surprises

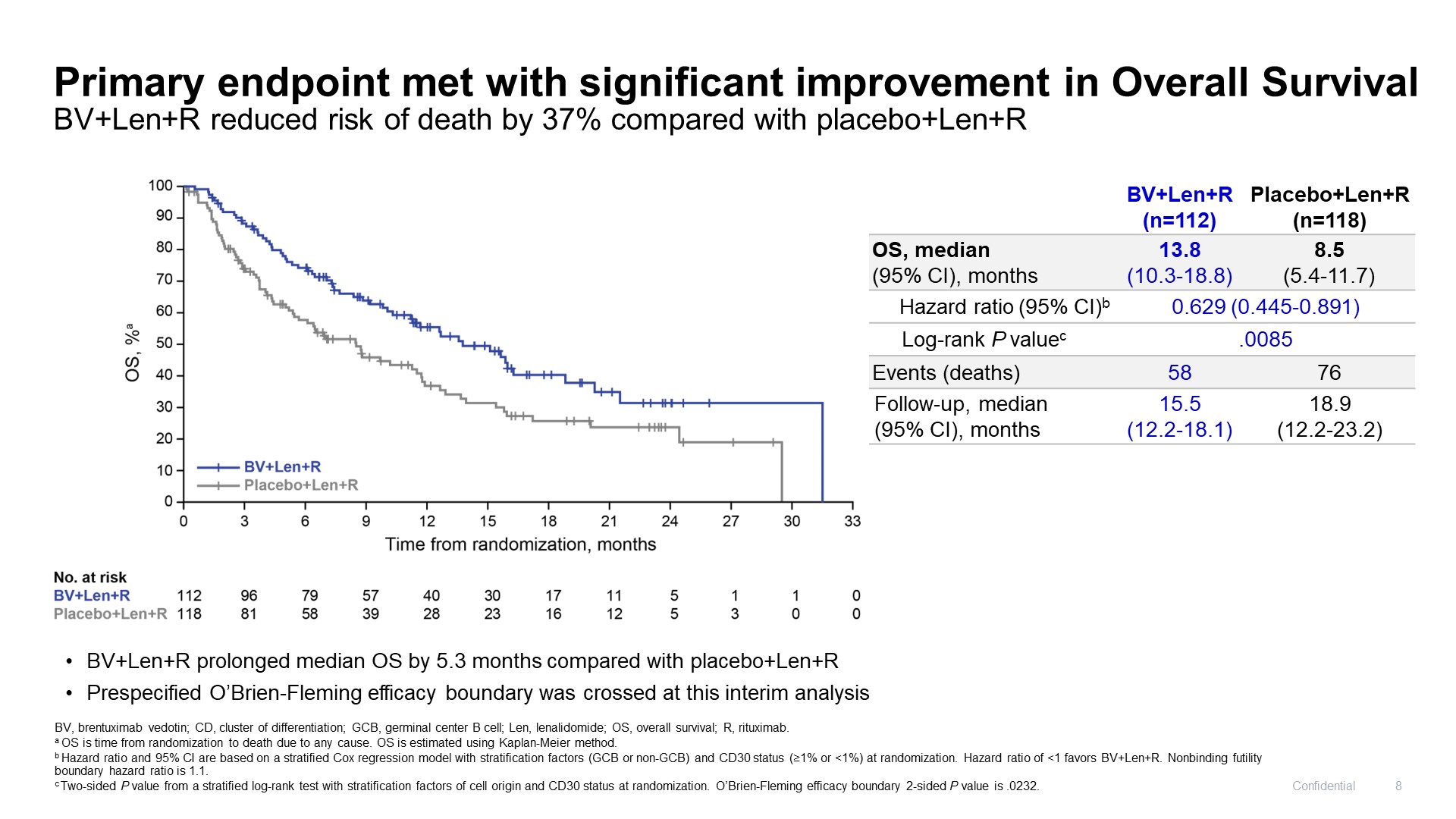
Adcetris’s Echelon-3 trial in diffuse large B-cell lymphoma delivered two more surprises at its ASCO late-breaker yesterday. After Pfizer toplined an OS win its ASCO presentation revealed a positive PFS result too, as well as strong data in patients whose cancers didn’t express meaningful levels of CD30, the antigen Adcetris targets. Echelon-3 combined Adcetris with Revlimid and Rituxan, and compared this against Revlimid and Rituxan, in third-line DLBCL ineligible for stem cell transplant or Car-T. Amazingly, the study had been deprioritised by Adcetris’s originator, Seagen, and deemed “no longer registrational”, with its enrolment cut and co-primary PFS endpoint relegated to a secondary measure. But in March Pfizer claimed a statistically significant OS benefit, now revealed as a 37% reduction in risk of death. Despite PFS’s relegation, the PFS benefit is also statistically significant, with a 0.53 hazard ratio despite only a 1.6-month benefit at median. 68% of Echelon-3’s population had CD30 expression of under 1%, yet risk of death in this group was reduced by 44%. Though this setting could give Adcetris its eighth approval, in the real world the drug will have to contend with Roche’s Polivy, whose bendamustine and Rituxan combo carries a third-line DLBCL label.
2653













