
ASCO 2024 – Pfizer’s KAT6 inhibitor purrs

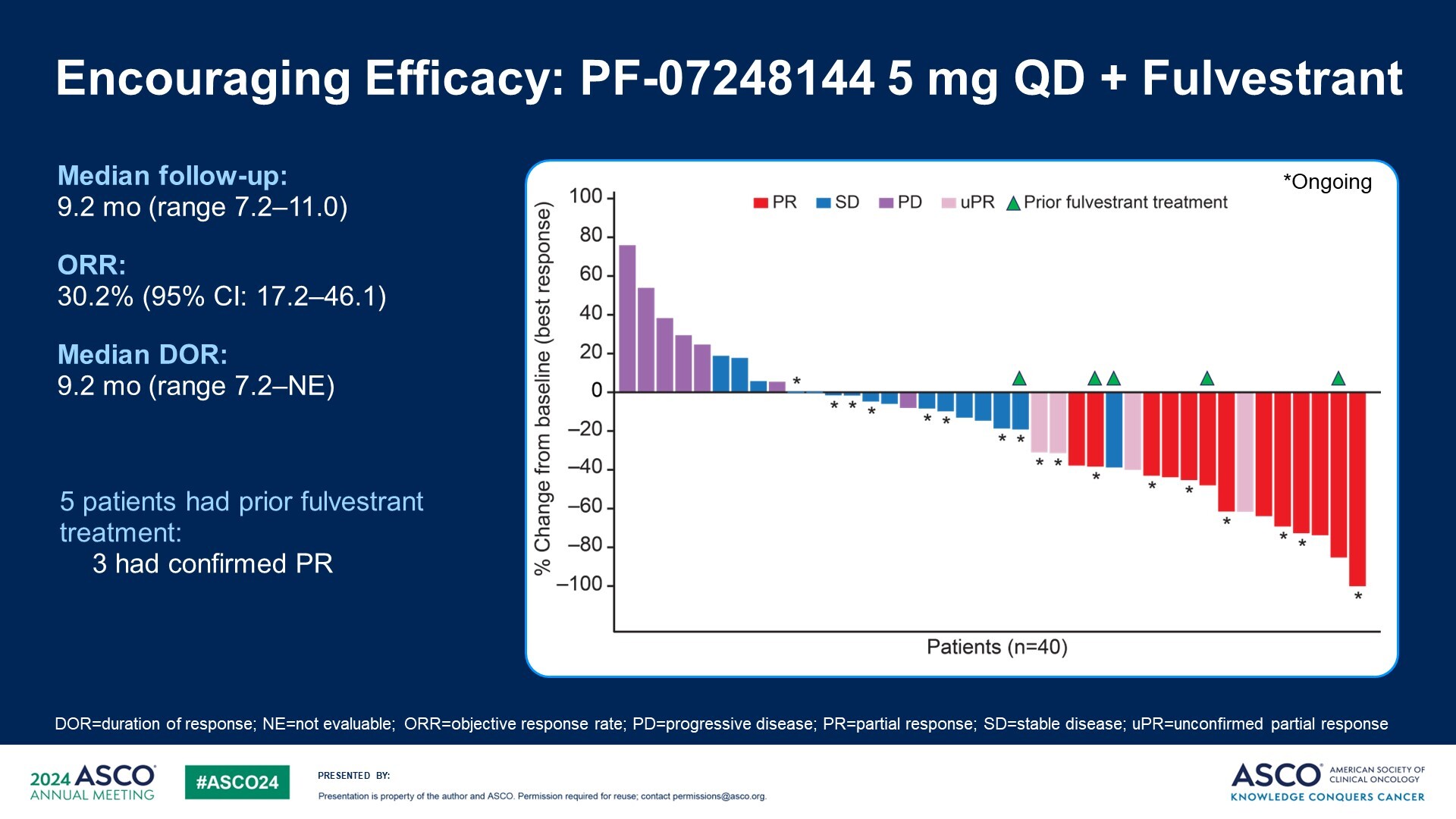
Pfizer is one of only a handful of companies developing a KAT6 inhibitor and its contender, PF-07248144, yesterday showed early promise in a phase 1 trial in ER-positive, HER2-negative breast cancer patients who had progressed on a CDK4/6 inhibitor. As per the ASCO abstract, the ORR was 11% with monotherapy and 30% with a Faslodex combo. But when presenting the data Dr Patricia LoRusso, of Yale University Cancer Center, mentioned that an updated data cut done in the “last few months” had yielded a combo ORR of 38%. She compared this with a historical 5% ORR with Faslodex alone in this setting. Particularly impressive were three partial responses among five patients previously treated with Faslodex who went on to receive the combo. Activity was also seen irrespective of ESR1 mutation status; SERDs like Faslodex have shown better efficacy in ESR1m patients. The most frequent adverse event was dysgeusia, or altered taste, while grade 3 or higher neutropenia was seen in 44% of combo patients; 7% of combo patients discontinued owing to an adverse event. Other KAT6 inhibitors in development include Olema’s OP-3136 and Menarini’s ISM5043, although all other projects are preclinical.
5434













