
ASCO 2024 – AbbVie sees SEZ6 improvement

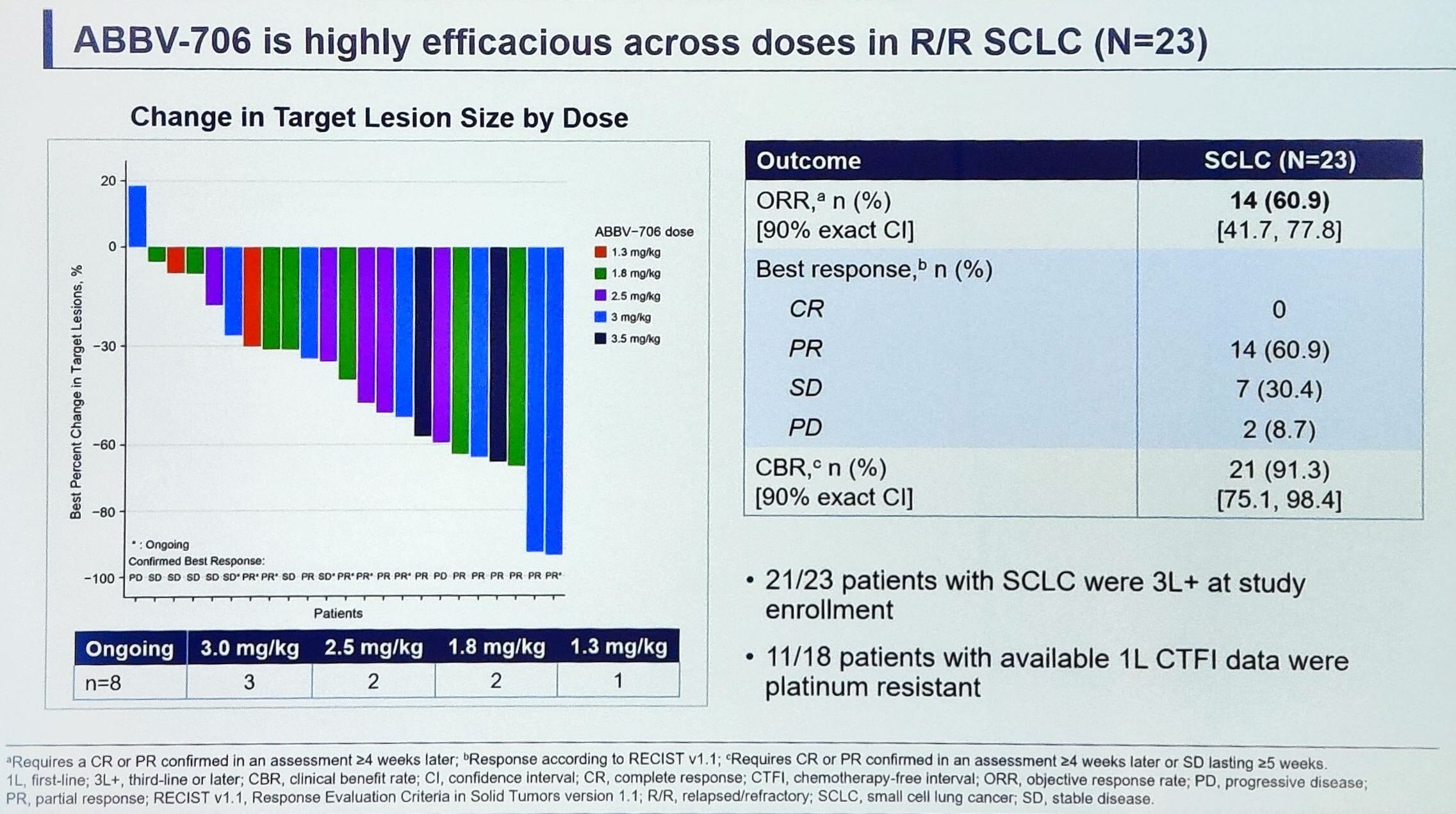
AbbVie’s SEZ6-targeting ADC ABBV-706 had already shown promising efficacy in small-cell lung cancer and neuroendocrine neoplasms in its ASCO abstract, and those figures improved during the full presentation today. The new confirmed overall response rate of 61% in second-line or later SCLC looks particularly impressive when compared – on a cross-trial basis – against the 40% seen with Amgen’s Imdelltra in this setting. As for side effects, anaemia and neutropenia were most common, with grade 3 or higher events seen in 42% of patients. As previously disclosed, there were two dose-limiting toxicities at 3.0mg/kg and 3.5mg/kg; with many responses seen at these doses, AbbVie will have to hope that it can tread the risk/benefit line. Dose interruptions and reductions were common, at 57% and 38% respectively, but only 4% of patients discontinued therapy. There were also seven deaths, but all were deemed unrelated to ABBV-706. AbbVie hasn’t disclosed which dose(s) it intends to take forward into dose expansion – a combination with its PD-1 inhibitor budigalimab is also planned.
Abbvie’s evolving SEZ6 dataset
| ASCO abstract | ASCO presentation | |
|---|---|---|
| Cutoff | 15 Nov 2023 | 20 Mar 2024 |
| ORR SCLC + neuroendocrine neoplasms | 21% (7/33) | 44% (21/48) |
| ORR SCLC | 40% (6/15) | 61% (14/23) |
| ORR neuroendocrine neoplasms | 6% (1/18) | 28% (7/25) |
Source: Dr Sreenivasa R Chandana & ASCO.
This story has been updated.
2434













