
ASCO 2024 – full Evoke-01 result adds to Gilead’s Trodelvy problems
Gilead came out swinging in second-line lung cancer, but it might have just whiffed.
Gilead came out swinging in second-line lung cancer, but it might have just whiffed.

After yesterday’s nasty surprise for Trodelvy – the drug failed a confirmatory bladder cancer trial, threatening its ongoing US approval – Gilead investors today got a second dose of bad news, from a late-breaking ASCO presentation on Trodelvy’s lung cancer study, Evoke-01.
Evoke-01, testing the anti-TROP2 ADC in second-line NSCLC, was formally a failure, but when Gilead toplined it in January it played up a survival benefit in patients who never responded to PD-(L)1 blockade, and said it would file on this. Today such hopes took a knock, when the full data revealed this subgroup claim to be a likely chance finding.
Gilead famously paid $21bn to acquire Trodelvy’s maker, Immunomedics, a move already shown to be rash by the drug’s underwhelming performance in breast cancer, and by its overshadowing by Daiichi Sankyo/AstraZeneca’s TROP2-directed ADC rival, datopotamab deruxtecan.
Gilead stock traded down 1% this morning. However, that was at least partly down to Trodelvy yesterday failing its phase 3 Tropics-04 trial in urothelial bladder cancer. The drug has US accelerated approval in this setting, based on the uncontrolled phase 2 Trophy U-01 study, and because Tropics-04 was a potentially confirmatory trial that approval now risks being revoked by the FDA.
Lung cancer
Clearly, however, NSCLC represents a larger use, which is why there had been some hope that Trodelvy might find a way into this market based on the subgroup benefit Gilead had touted as “clinically meaningful” in Evoke-01.
In the event, however, this seems illusory. Survival curves unveiled today for this subgroup cross over several times before separating later, and the absolute benefit – medians of 11.8 months versus 8.3 months for docetaxel control, with a 25% reduction in risk of death – come with a confidence interval upper bound very close to 1.00.
Evoke-01 overall survival in patients non-responsive to last PD-(L)1 therapy
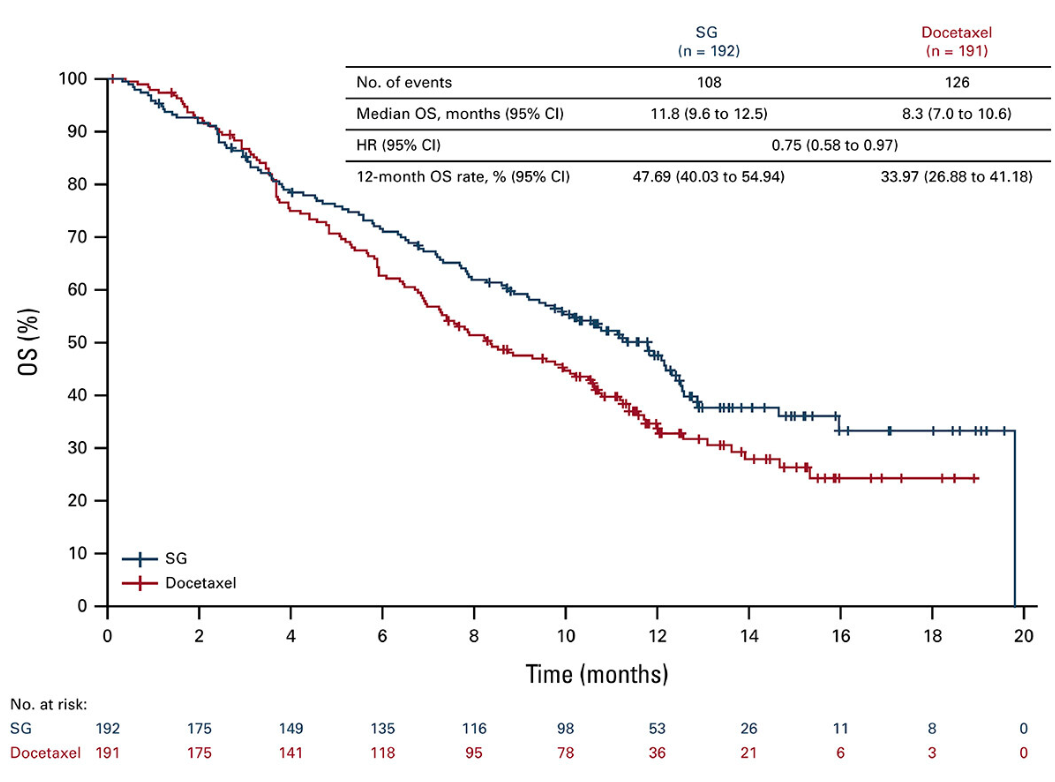
Potential reasons for cheer include that Trodelvy appeared to be safer than docetaxel in all-comers: treatment-emergent adverse events leading to discontinuation were 9.8% for the Gilead drug versus 16.7% for control, and those leading to death came in at 3.4% against 4.5%.
The all-comers overall survival benefit, Evoke-01’s primary endpoint, has been revealed as a very near miss for Trodelvy, whose hazard ratio of 0.84 versus docetaxel yielded a p value of 0.0534. But the numerical benefit, just 1.3 months, clearly lacks clinical meaningfulness, and on overall response rate patients on docetaxel actually did better, with 18.1% versus Trodelvy’s 13.7%.
Histology
A potentially more important consideration is histology, which has become a key battleground since dato-dxd succeeded in the second-line NSCLC study Tropion-Lung01 only in non-squamous patients.
Gilead has claimed that Evoke-01 results are seen irrespective of histology, and while the ASCO data bear this out the takeaway from all-comers is that neither histologies have yielded anything positive: median OS benefit is 1 month in squamous and 1.4 months in non-squamous patients, and both are clearly non-significant.
For their part Daiichi/Astra have filed dato-dxd in second-line NSCLC only in non-squamous patients, and the drug faces a December PDUFA date. Another clue about the importance of histology in this setting comes from a third anti-TROP2 ADC, the Kelun-derived sacituzumab tirumotecan, which is licensed in the west to Merck & Co.
Two Merck-sponsored sacituzumab tirumotecan phase 3 trial are under way in second-line NSCLC, one in patients with EGFR and other mutations, and the other in those progressed on tyrosine kinase inhibitors; tellingly, both target the non-squamous histology only.
Ultimately, with histology offering no solace in Evoke-01 and the Gilead-touted subgroup seeming illusory, Trodelvy is still far from justifying its $21bn price tag.
Cross-trial comparison of dato-dxd vs Trodelvy in 2nd-line NSCLC
Tropion-Lung-01 | Evoke-01 | |||
|---|---|---|---|---|
| OS | Interim: 12.4mth vs 11.0mth (HR=0.90, statistically non-significant) | 11.1mth vs 9.8mth (HR=0.84, p=0.0534) | ||
| PFS* | 4.4mth vs 3.7mth (HR=0.75, p=0.004) | 4.1mth vs 3.9mth (HR=0.92, statistically non-significant) | ||
| OS by histology | Non-squamous | Squamous | Non-squamous | Squamous |
| Interim HR=0.77, “clinically meaningful” at final analysis | Interim HR=1.32 | 11.3mth vs 9.9mth (HR=0.87) | 10.2mth vs 9.2mth (HR=0.83) | |
| OS by response to last PD-(L)1 | Non-responders | Responders | Non-responders | Responders |
| Not given | 11.8mth vs 8.3mth (HR=0.75) | 9.6mth vs 10.6mth (HR=1.09) | ||
Note: *PFS is a co-primary endpoint in Tropion-Lung01, and a secondary endpoint in Evoke-01. Source: ESMO 2023 & ASCO 2024.
2956













