
No purple patch for Purple Biotech

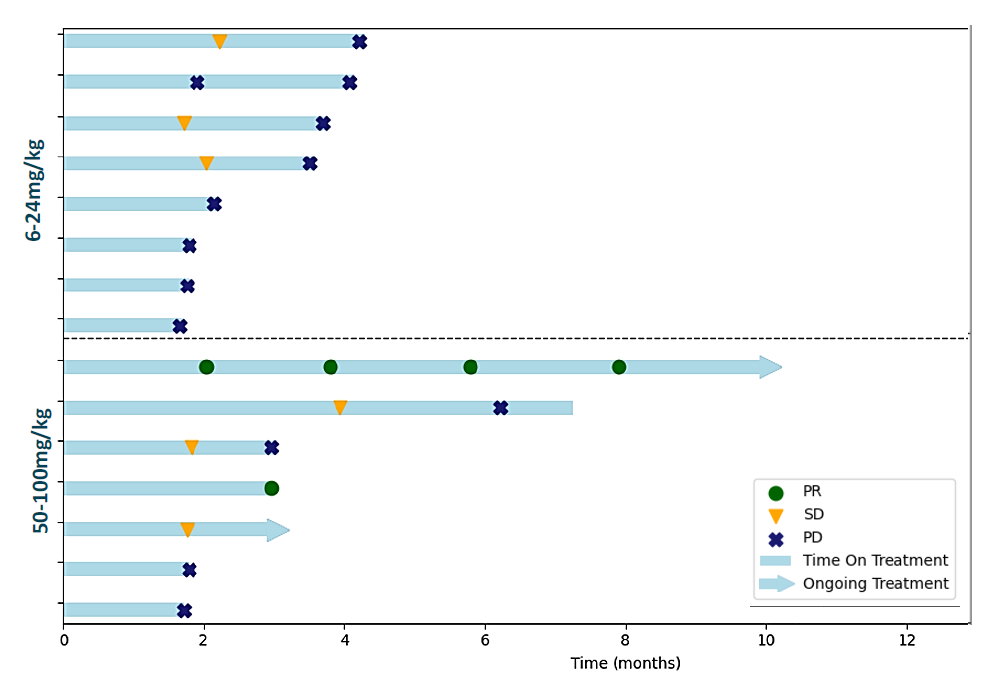
The little-known Israeli microcap Purple Biotech reckons it’s seen enough to take its IRS1/IRS2/STAT3 inhibitor NS219 into phase 2 in head and neck cancer. However, the backing for this remains sketchy: a phase 1 dose-escalation update, presented at this week’s ESMO Targeted Anticancer Therapies congress, shows two confirmed partial responses among 15 patients with median third-line head and neck cancer given NS219 plus Erbitux. Both occurred on 50mg/kg, and Purple claimed a 29% ORR among the seven patients given 50mg/kg or 100mg/kg. Erbitux alone in post-immunotherapy patients gives ORR of around 20%, and Jones Research says it was hoping for more robust efficacy; the analysts reckon Purple’s value driver is the anti-CEACAM1 MAb CM24, due to generate phase 2 pancreatic cancer data this year. Purple floated in 2015, when it was known as Kitov Pharma and focused on osteoarthritis pain/hypertension, since when its valuation has fallen to just $19m. OncologyPipeline shows plenty of STAT3 inhibitors, but NS219 is the only asset hitting IRS1 or IRS2. A phase 2 trial of NS219 (100mg/kg, which yielded no responses in phase 1) plus Erbitux in second-line head and neck cancer begins in the first half.
NT219 plus Erbitux in 2nd/3rd-line head & neck squamous cell carcinoma
885













