
ASCO-GU – picking apart Exelixis’s Contact-02 win
A rare success for Cabometyx sees a survival benefit, but the control regimen raises questions.
A rare success for Cabometyx sees a survival benefit, but the control regimen raises questions.

After failing to convince in the uncontrolled Cosmic-021 study Exelixis and Ipsen were hoping for a better reception to the results of Contact-02, a phase 3 trial in a broadly similar metastatic, castration-resistant prostate cancer setting.
Contact-02, testing Cabometyx plus Tecentriq in post-Zytiga/Xtandi patients, had been toplined as positive last August for its co-primary endpoint of progression-free survival versus Xtandi/Zytiga. However, the full data, revealed for the first time today at ASCO’s Genitourinary Cancers Symposium, raise questions about Cosmic-02’s design and the relevance of the survival hit.
At the heart of the issue lies Cosmic-02’s control regimen, which involved giving patients, who had already failed a novel hormonal therapy (Xtandi or Zytiga), another novel hormonal therapy; the usefulness of this so-called “hormone-switch therapy” is debatable. This is especially pertinent given that a chemotherapy, specifically docetaxel or Jevtana, was not an option for patients in Cosmic-02’s control arm.
Not only that, but just 23% of Contact-02 patients had received docetaxel in an earlier setting, and only 28% of control arm patients got chemo on progression, the ASCO-GU presentation revealed. Exelixis has argued that patients want an alternative to chemo – even though Contact-02 doesn’t test the Cabometyx regimen versus chemo – and today claimed that there were “limited options after progression on novel hormonal therapy”.
It’s true that many prostate cancer patients cannot tolerate or refuse chemo, but Cabometyx brings toxicities of its own. An alternative study protocol might have left up to the physician’s discretion what to prescribe the control cohort, though allowing only one option does make for a cleaner dataset.
Survival
As for the headline data, the PFS numbers have revealed medians of 6.3 months for Cabometyx plus Tecentriq, versus 4.2 months for second hormonal therapy. Risk of progression or death was cut by 35% (p=0.0007), and the presenting author, the Huntsman Cancer Institute’s Dr Neeraj Agarwal, called this clinically meaningful.
However, the ASCO-GU discussant, Dr Kim Chi of British Columbia Cancer Agency, disagreed, calling the PFS difference “modest” and saying it amounted to just one scanning cycle. He also highlighted the lack of an OS benefit – a key co-primary endpoint that is still immature at a median 12 months’ follow-up, but which was revealed for the first time.
At first sight median OS of 16.7 versus 14.6 months, and 21% reduction in risk of death, seems promising, but survival curves appear uninterpretable given that they overlap at least twice. A more robust sign of an OS benefit is thought to be vital for the chances of an approval based on Contact-02.
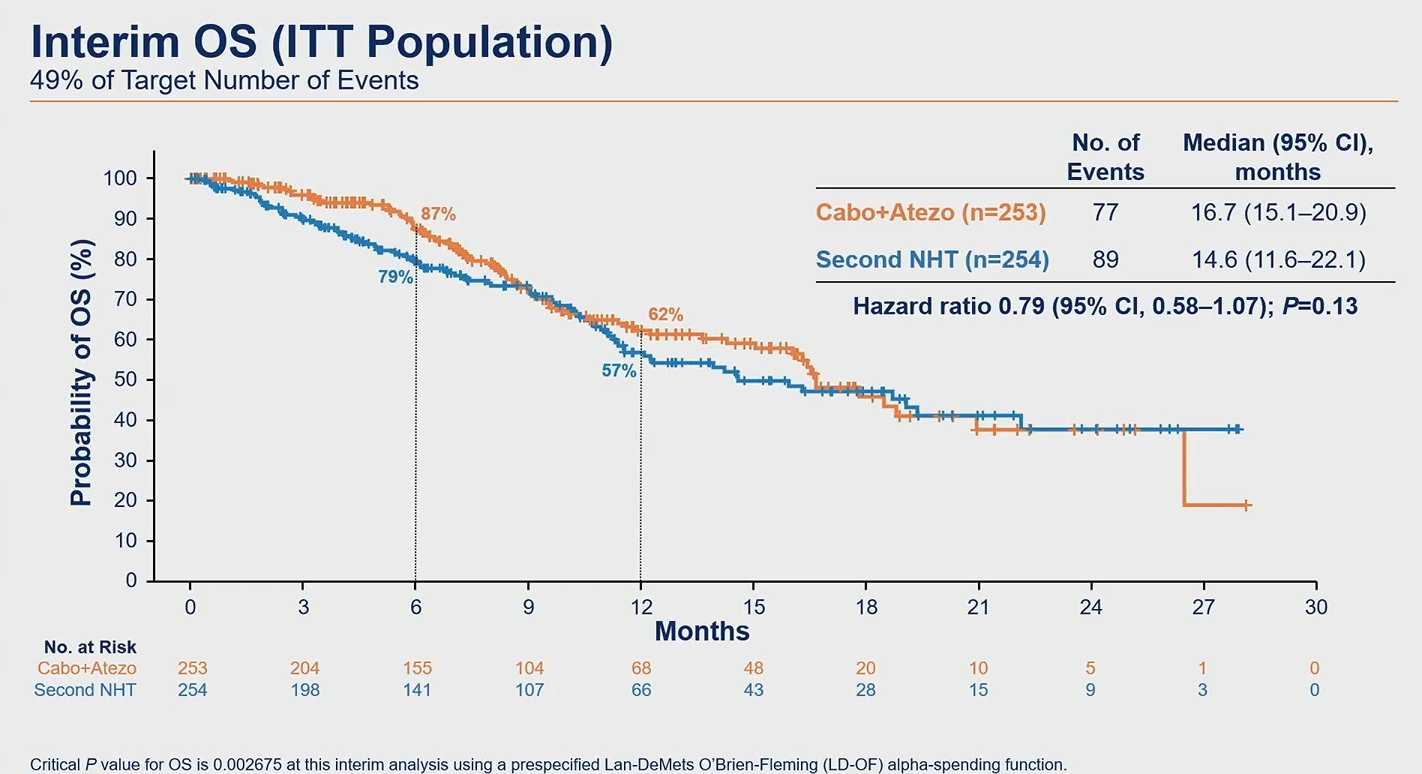
Source: Dr Neeraj Agarwal & ASCO.
The question of hormone-switch therapy is controversial in prostate cancer, and has recently featured in the broadly similar trials of two radiotherapeutics: Novartis’s PSMAfore and Point’s Splash. Both allowed crossover – albeit not to chemo but to active treatment – and in both cases the result was lack of an OS benefit.
Chi called out the non-availability of chemo in Contact-02’s control arm, and stated that hormone-switch was “not the best standard of care for this population”. He specifically cited docetaxel and Jevtana as viable and efficacious chemotherapies, and bemoaned the fact that just 28% of progressing patients in Contact-02’s control cohort did in fact go on to get chemo.
The Contact-02 data revealed a further headscratcher: prior docetaxel was a stratification factor in Cosmic-02, and the PFS data were also cut according to the resulting subgroups. This showed an especially pronounced survival benefit in patients who had received docetaxel – hazard ratios of 0.57 for PFS and 0.56 for OS (though this involved just 23% of Contact-02’s population).
Contact-02 is important for the future of Exelixis’s Cabometyx franchise, which beyond its blockbuster kidney cancer setting has yielded numerous study failures. Seeking approval in prostate cancer is a top 2024 priority for Exelixis, and the company will have to hope for better news when it presents a more mature OS analysis from Contact-02, promised later this year.
1732













