
FDA stops short of all-comers Keytruda label in cervical cancer

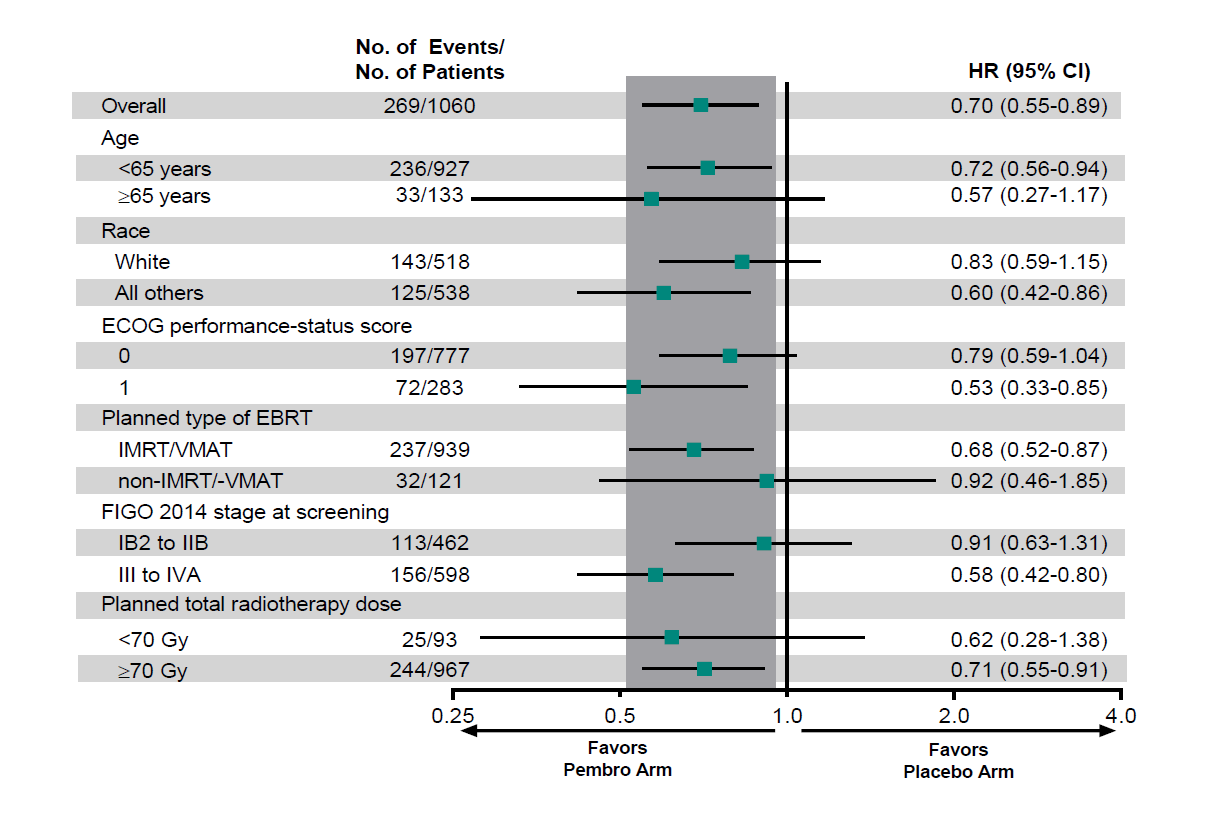
Last week’s US approval of Keytruda plus chemoradiotherapy for first-line cervical cancer broadens the Merck drug’s use in this setting, but it shows a still cautious stance from the FDA. The US regulator has declined to grant a label based on the all-comers population of the supporting Keynote-A18 trial, instead limiting approval to the 55% or so of patients who were stage III-IVA, according to FIGO 2014 criteria. Full Keynote-A18 data, presented as a late-breaker at last year’s ESMO conference, explain why: in stage III-IVA patients there was a 42% reduction in progression or death versus chemoradiotherapy alone, but in stage IB2-IIB the benefit was just 9%, with a hazard ratio upper bound of 1.31. Across the entire study the PFS endpoint was met, with a 0.70 hazard ratio (p=0.0020), while a 24-month landmark analysis of overall survival showed an immature numerical benefit favouring Keytruda (87.2% versus 80.8%). Until now the Merck drug’s label in first-line cervical cancer was limited to ≥1% PD-L1 expressers as part of a chemo combo, with or without Avastin, based on the Keynote-826 study; this also served as the confirmatory trial for Keytruda’s second-line approval, as monotherapy in ≥1% PD-L1 patients.
PFS analysis in protocol-specified subgroups of Keynote-A18
This story has been updated to correct a hazard ratio.
1026













