
ASH 2023 – Sympatico gives Imbruvica a second chance

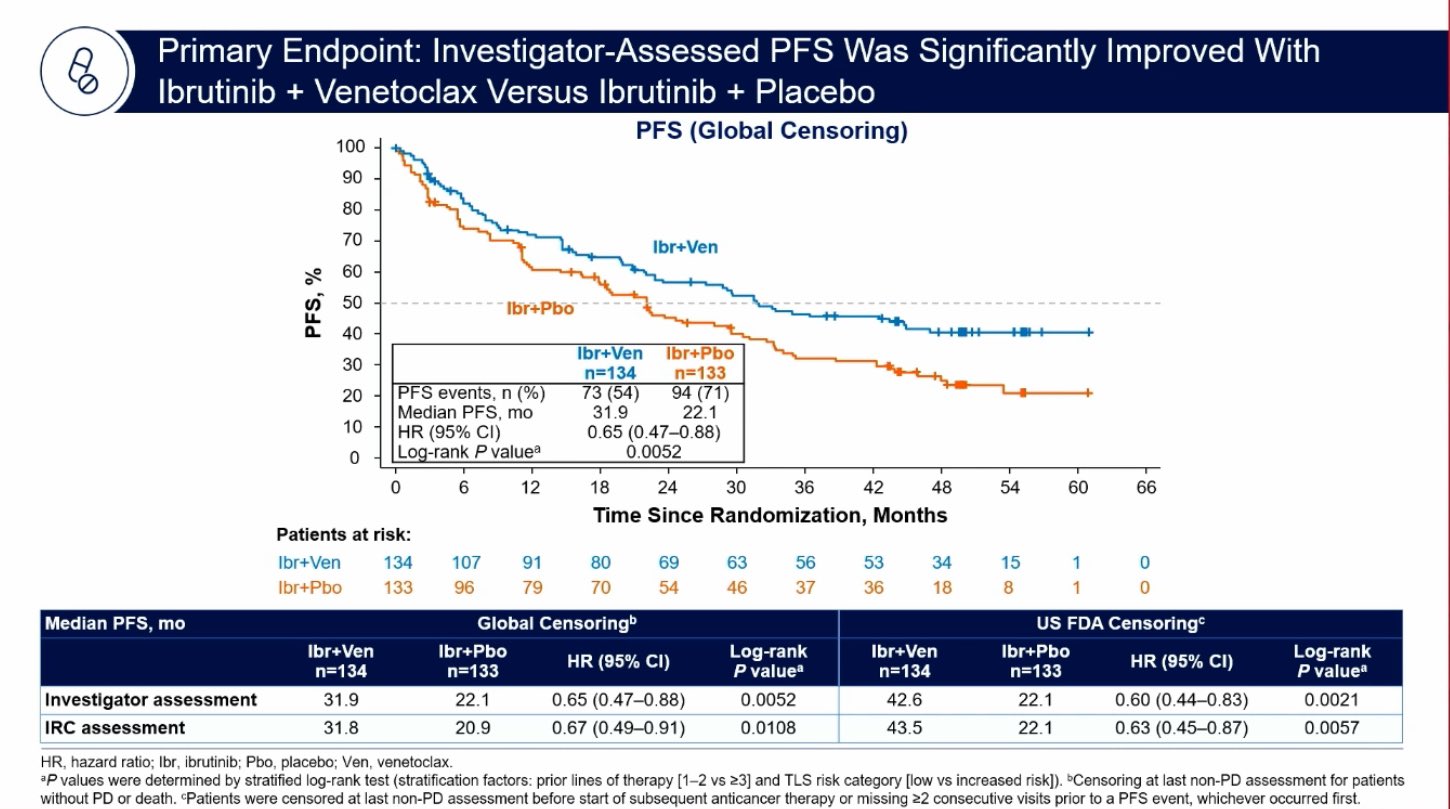
Mantle cell lymphoma is small fry compared with the big indication of chronic lymphoblastic leukaemia, explaining why AbbVie was able to shrug off the withdrawal of Imbruvica’s US approval in the former setting in April. But the phase 3 Sympatico study, presented as an ASH late breaker this week, could see Imbruvica return to MCL as part of a combination with Venclexta, which itself is a blockbuster in CLL. Imbruvica’s MCL nod had come on an accelerated basis, and withdrawal was prompted by contradictory results of the potentially confirmatory phase 3 Shine and Selene studies. Sympatico has shown a PFS benefit for Imbruvica plus Venclexta versus Imbruvica alone, and one obvious question, given Imbruvica’s withdrawal, is whether such a control cohort is viewed as appropriate. That said, a 74% remission rate for the control compares favourably with the 66% that Imbruvica’s pre-withdrawal label had cited. Another question is whether PFS will suffice without a clear OS benefit; the latter is numerically positive after 144 deaths, and the lead investigator, MD Anderson’s Dr Michael Wang, told an ASH press briefing that about 170 will be needed to give sufficient powering for a mature OS readout, expected in early 2025.
768













