
ASH 2023 – lacutamab’s late-line promise on hold

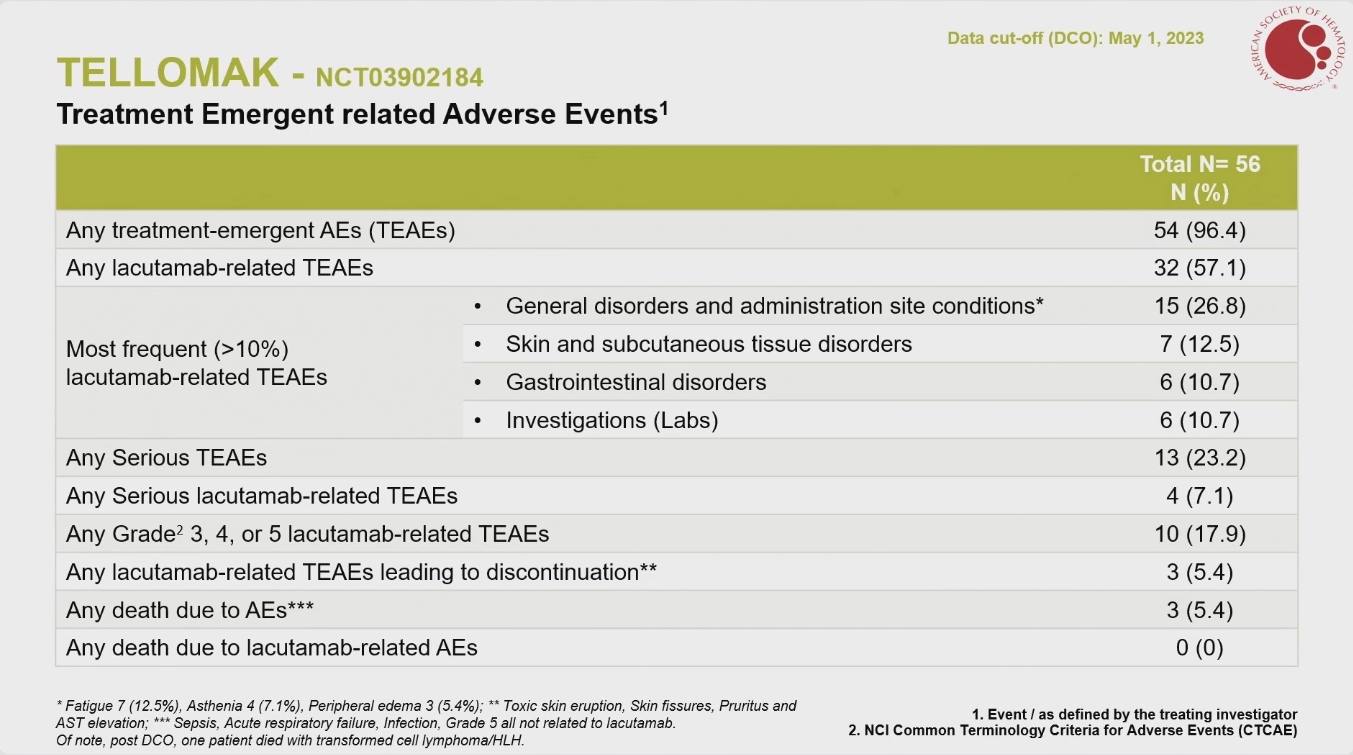
ASH attendees heard on Saturday that Innate Pharma’s lacutamab could be a good option in late-line Sézary syndrome, a cutaneous T-cell lymphoma with high unmet need. However, the presenter, Professor Pierluigi Porcu of the Sidney Kimmel Cancer Center, didn't mention that this project was on partial clinical hold after a fatal case of haemophagocytic lymphohistiocytosis. Indeed his presentation, which covered cohort 1 of the phase 2 Tellomak trial, highlighted zero deaths due to lacutamab-related adverse events; a footnote on the same slide noted that one patient died after data cutoff on May 1, 2023. As for efficacy, lacutamab produced an overall response rate of 38% among 56 patients, in line with the ASH abstract; subjects had received a median of five prior therapies including mogamulizumab, one of the few specific treatments for Sézary, which involves malignant T-cells attacking the skin. Innate has scheduled an investor event for Tuesday, so perhaps its next steps will become clearer then. Tellomak also includes three other cohorts evaluating lacutamab in mycosis fungoides, another type of cutaneous T-cell lymphoma. The asset, the only one in development targeting KIR3DL2, according to OncologyPipeline, is also in phase 1 in peripheral T-cell lymphoma.
Adverse events with lacutamab
676













