
ESMO 2023 – BioNTech still has work to do on Claudin6

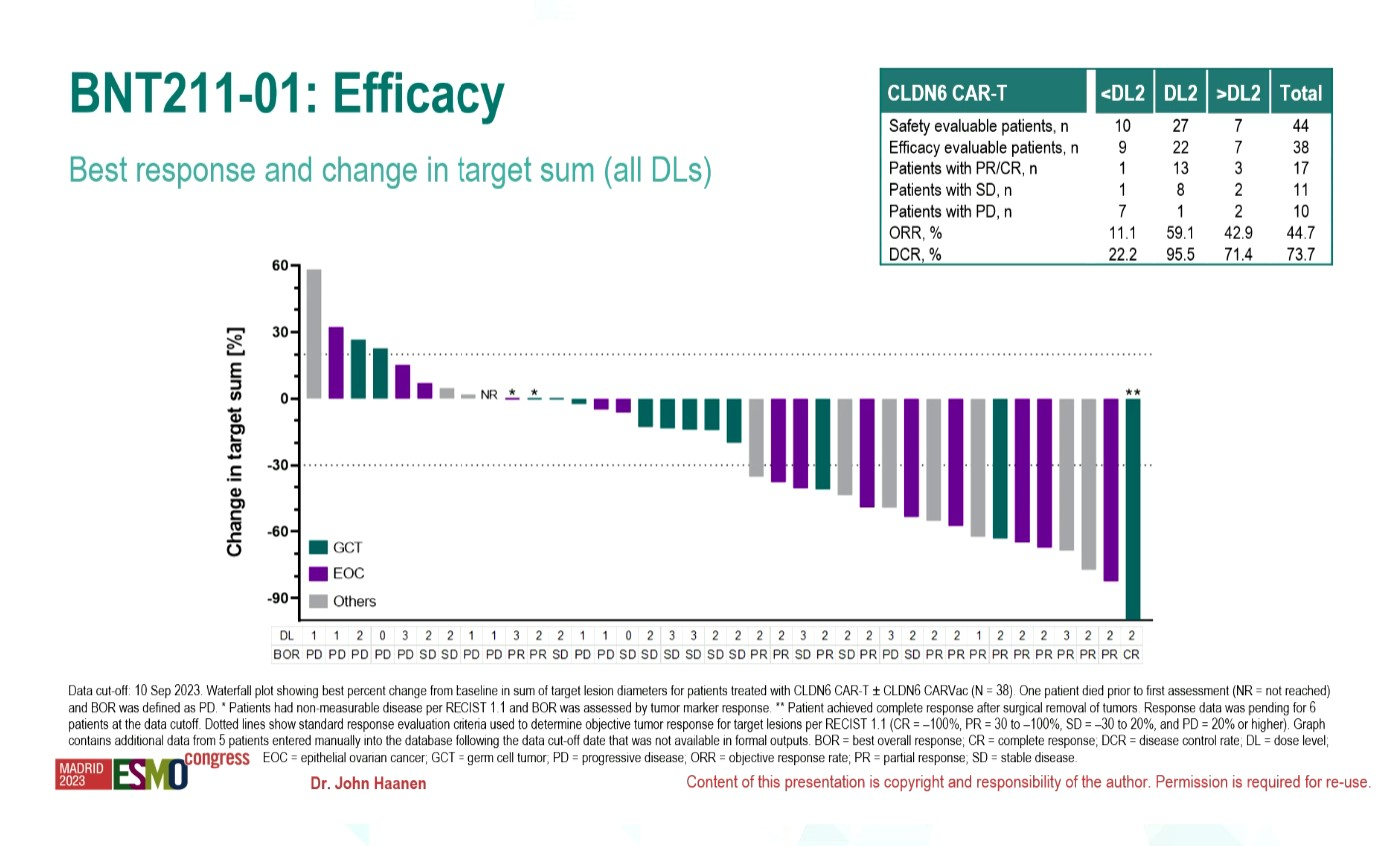
On the face of it a 59% response rate with BioNTech’s Claudin6-targeting Car-T therapy BNT211 seems promising. But a closer look at the results presented at ESMO yesterday, from a phase 1/2 trial in Claudin6-positive solid tumours, shows that this was achieved with a dose of 1x108 Car-T cells that “seems to be toxic”, according to the discussant, Centre Léon Bérard’s Dr Philippe Cassier. 15 of 27 patients receiving this dose experienced cytokine release syndrome. Across the whole trial, the ORR was 45% among 38 evaluable patients – still a respectable result in a study population dominated by ovarian and germ cell cancers. The investigators are now “backfilling” doses to find a recommended phase 2 dose. After last year’s ESMO update covered a manual manufacturing process yesterday's data involved automation, which adds potency and toxicity alike. Some patients also received a mRNA vaccine, called CarVac, designed to boost BNT211. The ESMO presentation didn’t break out responses according to who had received CarVac, although biomarker data suggested that the vaccine could improve BNT211’s persistence. BioNTech still has much to iron out ahead of its phase 2 trial of BNT211, set to begin next year. Still, the company is ahead in the Claudin6 arena.
| Project | Company | Description | Status | Note |
|---|---|---|---|---|
| BNT211 | BioNTech | CLDN6 CAR-T | Ph1/2 trial +/- CARVac in CLDN6+ solid tumours (NCT04503278) | ESMO 2023 with automated manufacturing: 45% ORR (17/38) across all doses; 59% ORR (13/22) at dose level 2 (1x108 Car-T cells) |
| TORL-1-23 | TORL Biotherapeutics | Anti-CLDN6 ADC | Ph1 in solid tumours (NCT05103683) | ASCO 2023: 28% ORR (7/25) across all doses |
| DS-9606 | Daiichi Sankyo | Anti-CLDN6 ADC | Ph1 in tumours known to express CLDN6 (NCT05394675) | Primary completion Nov 2023 |
| BNT142 | BioNTech | Anti-CLDN6/CD3 mRNA-encoded bispecific MAb | PH1/2 in CLDN6+ solid tumours (NCT05262530) | Primary completion Oct 2025 |
| AMG 794 | Amgen | Anti-CLDN6/CD3 BiTE | Ph1 in CLDN+ NSCLC, epithelial ovarian cancer & other solid tumours (NCT05317078) | Primary completion Apr 2026 |
| SAIL66 | Roche/ Chugai | Anti-CLDN6/CD3 bispecific antibody | Ph1 in CLDN+ solid tumours (NCT05735366) | Primary completion Dec 2028 |
Source: OncologyPipeline.
2966













