
ESMO 2023 – sabestomig has it all to do in TIM-3
Astra manages to avoid the toxicities that scuppered Lilly’s PD-1 x TIM-3 contender, but still needs to dial up sabestomig’s efficacy.
Astra manages to avoid the toxicities that scuppered Lilly’s PD-1 x TIM-3 contender, but still needs to dial up sabestomig’s efficacy.

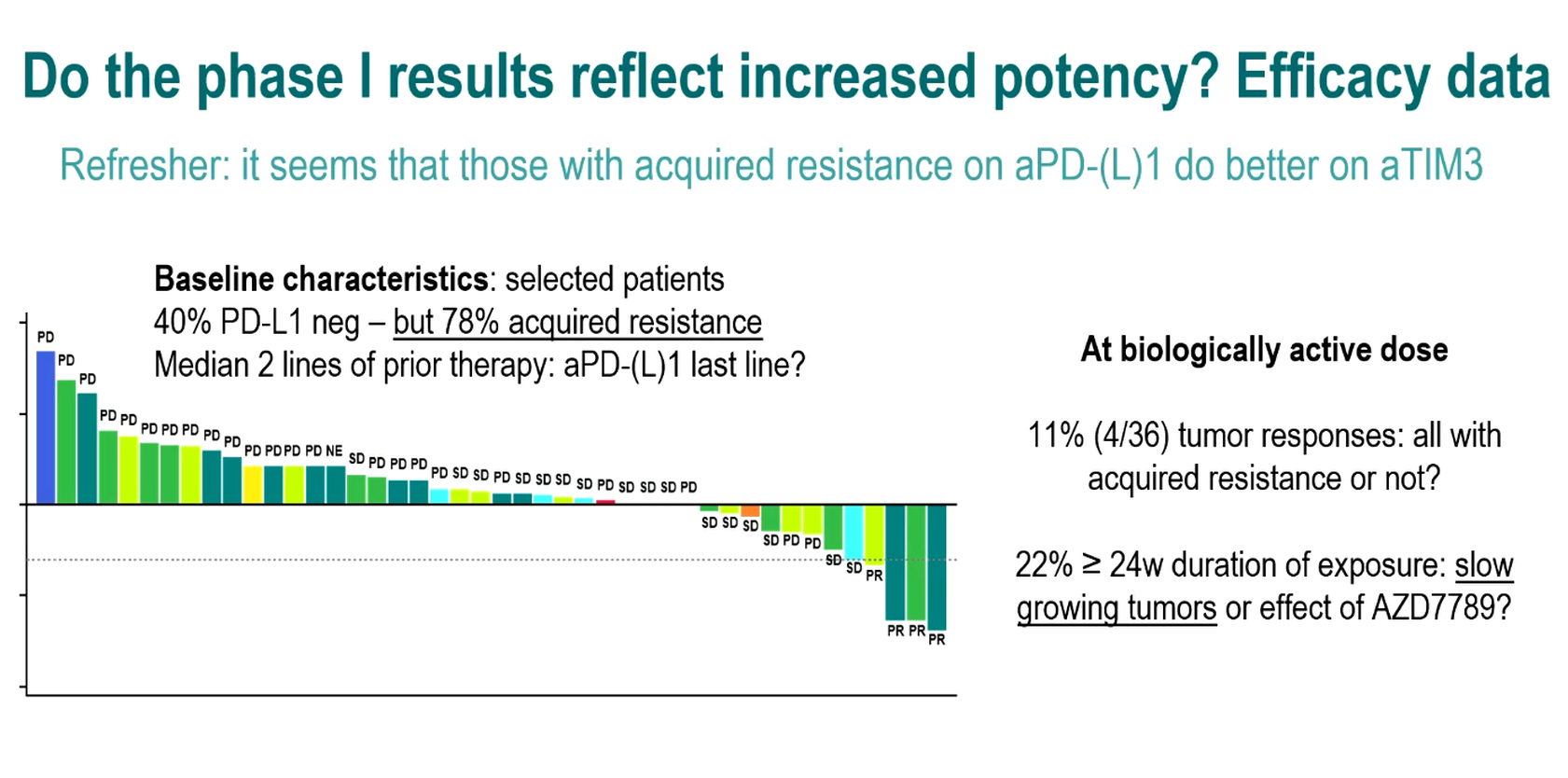
It might be a measure of the intractability of lung cancer once patients relapse on chemo and PD-(L)1 blockade that an 11% response rate justifies pressing on with a project clinically. This appears to be where AstraZeneca finds itself with its PD-L1 x TIM-3 bispecific sabestomig, based on phase 1 data presented at ESMO this morning.
The sabestomig first-in-human results are relevant for other TIM-3 players, particularly GSK and Novartis, and on the plus side Astra has avoided the toxicity that scuppered Lilly’s rival bispecific. Still, as Astra has already shown at ESMO in the separate Tropion-Lung01 study, much better efficacy is needed to beat docetaxel, the second-line NSCLC standard.
There was, of course, no docetaxel comparator in the uncontrolled sabestomig trial presented at ESMO, where confirmed partial remissions were seen in four of 36 patients given doses of 750mg or above. The responding patients had failed two to six therapy lines, and two were PD-L1 negative.
What’s the comparison?
Though Tropion-Lung01 tests an entirely separate Astra project, the Daiichi Sankyo-partnered TROP2 ADC datapotamab deruxtecan, that study involves a very similar NSCLC setting so presents a handy cross-trial comparison. Here, an ESMO late breaker showed that docetaxel alone can yield 13% ORR.
Not only that, but a seemingly promising 26% ORR for datopotamab in this trial has translated into under a month’s extra median PFS – a result that probably lacks clinical meaningfulness and offers a sobering reminder of just how far sabestomig has to go to make a mark.
Source: Dr Lizza Hendriks & ESMO.
Gustave Roussy’s Professor Benjamin Besse told ESMO that enrolment would now be pursued into two sabestomig NSCLC expansion cohorts, the first in IO-naive patients expressing PD-L1 at ≥50%, and the second in PD-L1 ≥1% expressers with IO acquired resistance in the second-line setting. No details are available on clinicaltrials.gov yet.
The study’s discussant, Dr Lizza Hendriks of Maastricht University Medical Center, suggested that a future trial in the first setting should compare sabestomig monotherapy versus an anti-PD-L1, perhaps focusing on yet to be defined biomarkers. The second-line setting, meanwhile, left open the question of what the comparator would be, and could change if a project like datopotamab managed to displace docetaxel.
Safety
Whatever questions remained about lack of efficacy, Hendriks praised sabestomig’s safety profile, which revealed low rates of severe adverse events, and just one grade 3+ event deemed treatment related, among all 45 patients dosed. At least this leaves open the possibility of shooting for better efficacy by dosing sabestomig higher.
This is relevant considering the experience of Lilly, which discontinued the anti-PD-1 x TIM-3 bispecific LY3415244 after seeing a 17% rate of anaphylactic infusion-related reactions and the development of antidrug antibodies.
OncologyPipeline shows sabestomig to be the only PD-1 x TIM-3 bispecific still in development, Roche having also discontinued its contender, lomvastomig, last year. Meanwhile, the key monospecific anti-TIM-3 MAbs are Novartis’s sabatolimab and GSK’s cobolimab, with Lilly having discontinued LY3321367.
Hendriks pointed out the absence of monotherapy activity sabatolimab and cobolimab had shown (LY3321367 seemed inactive even in combination with PD-L1 blockade), a fact that explains why both are now in combo trials. However, as Novartis is no longer pursuing NSCLC with sabatolimab, perhaps the most relevant mechanistic competitor for sabestomig is GSK’s cobolimab plus Jemperli in the phase 2/3 Costar Lung study.
Sabestomig offers the promise of both mechanisms in a single molecule, but whether Astra has more luck here than Lilly or Roche has yet to be determined.
1747













