
ESMO 2023 – Perla gives Jemperli combos more backing
The numerical survival benefit versus Keytruda underperform’s Merck’s phase 3 study, but there’s a possible reason for that.
The numerical survival benefit versus Keytruda underperform’s Merck’s phase 3 study, but there’s a possible reason for that.

GSK’s anti-PD-1 laggard Jemperli has received a significant boost not only from its Perla trial against Keytruda, whose overall survival curves were just presented at ESMO, but also from supportive comments by the late-breaker’s discussant addressing possible criticism of the result.
The front-line lung cancer trial’s biggest failing is its small size, comprising just 243 patients in total, but the ESMO data marked another success after Perla yielded positive remission rate results across various cuts of PD-L1 expression. Overall, the data support the use of Jemperli as a backbone for immunotherapy combos, though it’s up to GSK to make the most of any resulting advantage.
The phase 2 study, pitting chemo combos of Jemperli and Keytruda head to head in first-line non-squamous NSCLC, had already shown Jemperli’s non-inferiority on ORR, with a numerical benefit to boot, and the ESMO abstract revealed a similar showing for OS. Today Professor Solange Peters, of University Hospital of Lausanne, presented fairly consistent survival curves.
Underperformance?
Nevertheless, one glaring fact is that Keytruda’s mOS of 15.9 months was much lower than the 22.0 months in this setting’s benchmark pivotal trial, Keynote-189.
But is it a fair comparison? The discussant, University of Barcelona’s Dr Noemi Reguart, opined that this was neither an underestimate of Keytruda’s efficacy in Perla nor an overestimate in Keynote-189, but rather the result of Perla including a greater share of PD-L1-negative patients.
These patients are logically expected to derive a smaller benefit from PD-(L)1 therapy than PD-L1 expressers, and made up 42% of the Keytruda control arm in Perla, versus just 31% in Keynote-189’s active cohort. Not only that, but Reguart pointed out that a slight imbalance in Perla patients’ baseline status for brain or liver metastases actually favoured the Keytruda cohort.
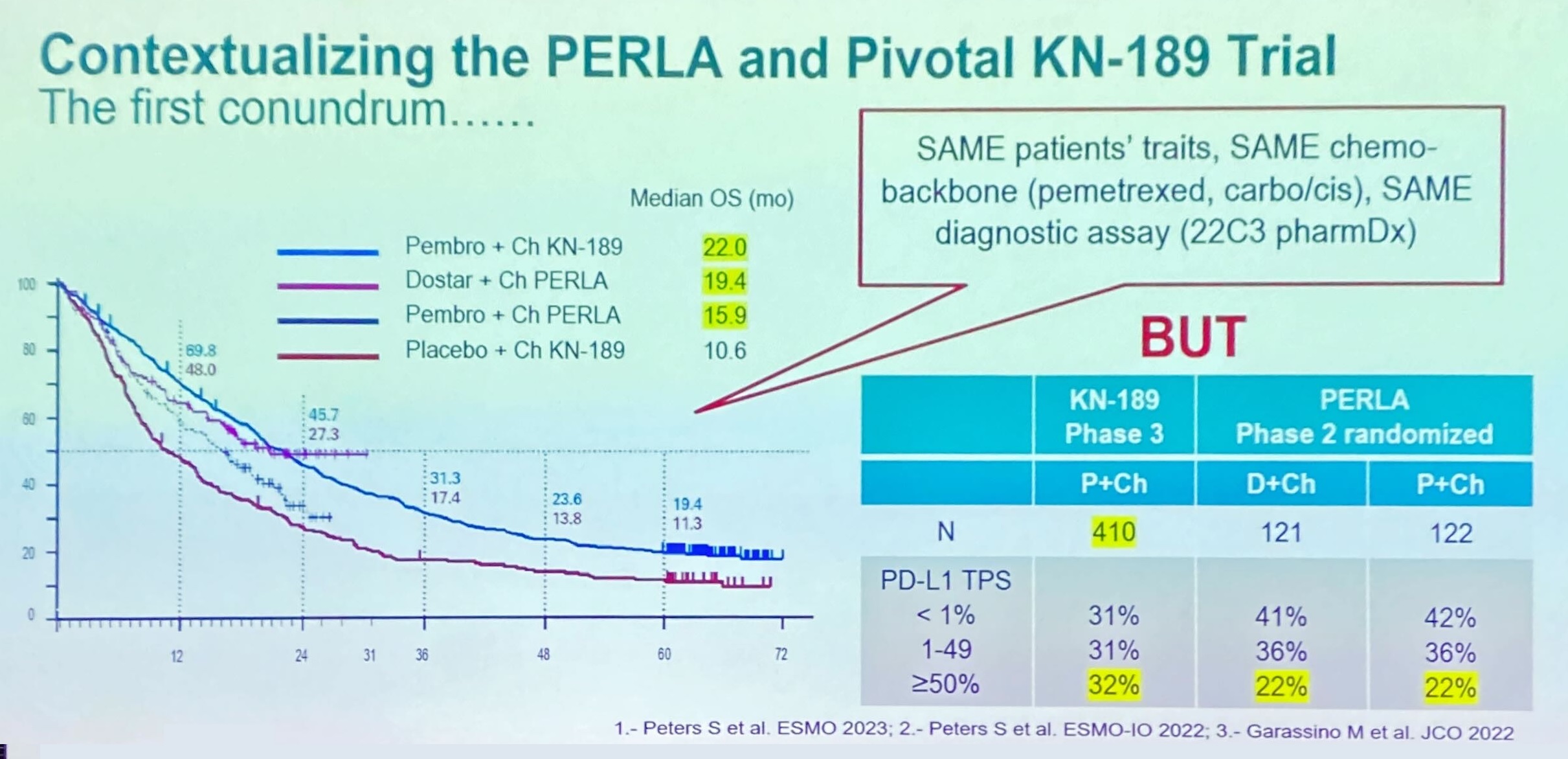
Patients in Perla were stratified by PD-L1 status, and Peters noted that the study’s proportion of PD-L1 <1% expressers was higher than in the real world.
She also argued that Jemperli featured important differences versus Keytruda, especially in terms of its binding profile, hitting different binding sites on PD-1, though she admitted that the precise clinical impact of this had yet to be determined.
The Perla data are important not specifically for Jemperli, which is a relative latecomer, but for determining the relevance of the results of studies that combine the GSK drug with other novel agents, in particular the anti-TIGIT MAb belrestotug, licensed from iTeos.
Peters and Reguart agreed that Perla supported Jemperli’s use as a treatment backbone, and showed that its efficacy was broadly equivalent to other landmark trials.
1041













