
Triple meeting 2023 – no monotherapy activity for Repare

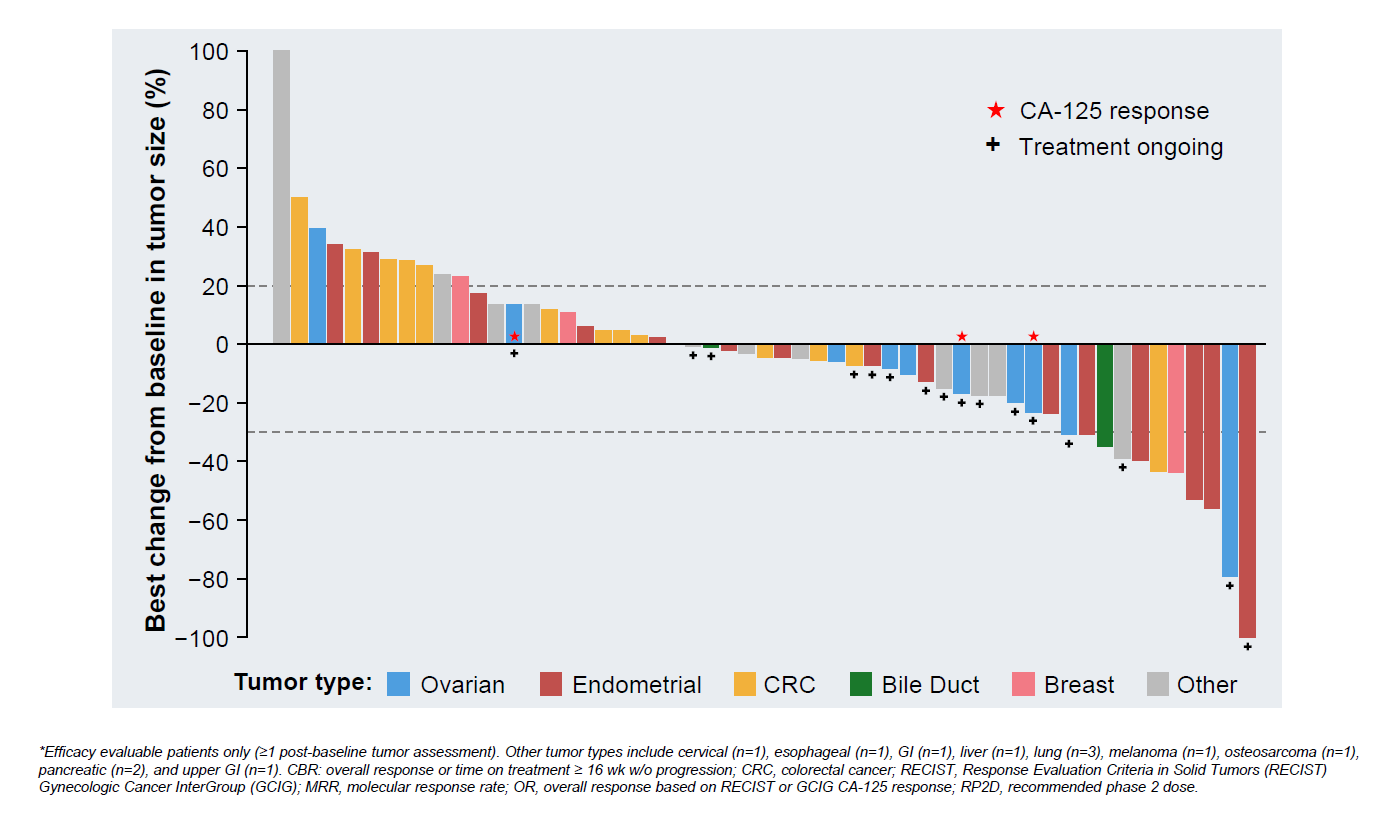
It’s hard to discern much from small share price movements in a biotech bear market, so perhaps Repare’s 6% fall on Friday is a relatively positive response to early results on the group’s PKMYT1 inhibitor lunresertib. Still, the data, presented at the Triple (EORTC-NCI-AACR) meeting, confirm that the only hope for lunresertib will come from combinations, as monotherapy yielded just one RECIST response among 67 treated subjects. Instead, Repare talked up a 24% response rate among 55 evaluable patients given lunresertib plus camonsertib, an ATR inhibitor it had licensed to Roche for $125m up front in 2022. PKMYT1 and ATR inhibition are two examples of synthetic lethality, and the mechanisms were said to act synergistically. Repare compared the combo against lunresertib monotherapy, enthusing about how activity was “enhanced” by adding one project to the other, but of course nothing is known about how much efficacy camonsertib alone accounts for. And a further problem is anaemia, seen in 68% of combo recipients, including 42% at grade 3. Nevertheless, Tapistry, a phase 2 lunresertib plus camonsertib trial, is to start by the end of 2023. OncologyPipeline lists Allist and Acrivon as the only other PKMYT1 players, both preclinical.
Lunresertib plus camonsertib in phase 1 Mythic study
This story has been amended to correct the mechanism of action of camonsertib.
1454













