
Olema follows Pfizer into KAT6

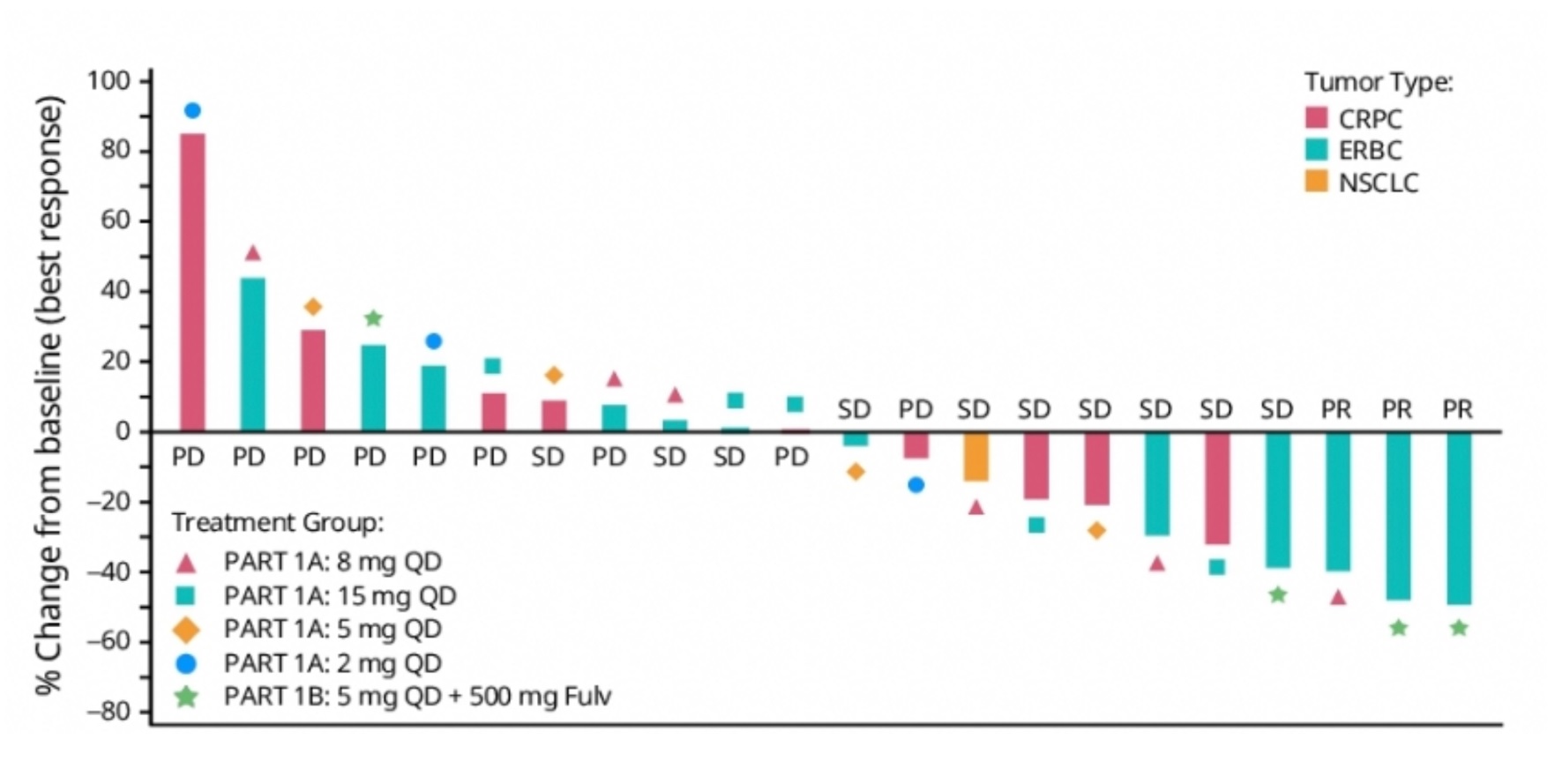
Not long after Pfizer presented the first human data with a KAT6 inhibitor at ASCO, Olema has unveiled its plans in this space. The latter company’s work on KAT6 inhibitors – no lead has apparently been selected yet – will feature at a poster at next month’s AACR-NCI-EORTC “Triple” meeting. The work stems from a June 2022 deal worth $8m up front, under which Olema gained rights to a project from Dr Reddy’s Aurigene subsidiary, now disclosed as concerning KAT6 inhibition. KAT6A and B are histone acetyltransferases that regulate gene transcription, whose disruption is thought to drive some oncogenic gene expression. Since Olema’s lead asset is the breast cancer SERD palazestrant the company's activity here makes sense: it was in ER-positive HER2-negative breast cancer that Pfizer’s PF-07248144 showed three partial remissions said to be confirmed and durable, among 12 evaluable subjects, according to a poster at ASCO. Two of those responses were in combination with Faslodex, but there were no remissions in 10 other patients, who had NSCLC or prostate cancer. OncologyPipeline reveals no other KAT6 inhibitors in development. Olema hasn’t said when it plans to enter the clinic, but Pfizer has promised an update on PF-07248144 next year.
Efficacy of Pfizer's KAT6 inhibitor PF-07248144
1592













