
World Lung 2023 – Flaura2 leaves a gap open for J&J
AstraZeneca’s trial lives up to its billing, though the benefit might be subgroup-driven, and comes at the cost of toxicity.
AstraZeneca’s trial lives up to its billing, though the benefit might be subgroup-driven, and comes at the cost of toxicity.

Johnson & Johnson’s nervous wait is over. With late-breaking results from AstraZeneca’s Flaura2 trial of Tagrisso plus chemotherapy just revealed at World Lung, J&J can probably rest assured that Tagrisso will remain the standard of care for front-line EGFR-mutated lung cancer for some time to come.
The issue was relevant because Tagrisso is the active comparator in J&J’s Mariposa trial of Rybrevant plus lazertinib. Of course, nothing is certain about Mariposa – that trial has passed an interim analysis without being halted for efficacy – and the Flaura2 data could in any case prompt a change of thinking about targeted treatment for certain NSCLC patients.
Flaura2 had already been toplined positive for progression-free survival. But, while hailing the PFS data as showing a “remarkable improvement”, and suggesting that the trial would have a profound impact on treatment, presenters at World Lung stopped short of calling the results practice changing.
Indeed, the discussant at today's presidential session, Guangdong Lung Cancer Institute's Professor Yi-Long Wu, said the Flaura2 regimen should not yet be considered standard of care in first-line EGFR-mutant NSCLC, because of the lack of an overall survival benefit.
Subgroup efficacy
Another issue is that the PFS result looks to be driven by a specific subset of patients – those with brain metastases at baseline. And adding chemo on top of Tagrisso brings with it a heavy toxicity price.
Tagrisso is already a firmly established standard in first-line EGFR-mutant NSCLC, on the basis of a PFS benefit versus the older drugs Iressa or Tarceva in the Flaura trial. Flaura2 had Tagrisso as control, and this performed in line with Flaura; adding chemo on top, meanwhile, extended median PFS by 9.5 months, Professor Pasi Jänne told World Lung’s presidential symposium today.
Flaura2 and Flaura trial results summarised
Tagrisso + chemo | Tagrisso monoRx | Iressa or Tarceva | |
|---|---|---|---|
| Flaura2 mOS | Not reached | Not reached | – |
HR=0.90 (27% maturity) | – | ||
| Flaura mOS | – | 38.6mth | 31.8mth |
– | HR=0.80 | ||
| Flaura2 mPFS | 29.4mth | 19.9mth | – |
HR=0.62 | – | ||
| Flaura mPFS | – | 18.9mth | 10.2mth |
– | HR=0.46 | ||
Source: IASLC & prescribing information.
So far so good. However, Flaura2’s safety might give pause: incidence of grade 3 or greater adverse effects rose from 27% with Tagrisso to 64% with the chemo combo, that of serious adverse events from 19% to 38%, and that of treatment-related serious adverse events from 5% to 19%. Neutropenia and thrombocytopenia stood out as materially increased.
The safety profile was “manageable with standard medical practice”, World Lung's presidential symposium heard today. But Wu suggested that what was needed was a combo with "more efficacy and less toxicity". Given the toxicity burden, regulators and doctors might in future want to see Tagrisso plus chemo kept back for only those patients in whom a benefit is clearcut.
One such group might be patients with brain metastases, who represented roughly 40% of Flaura2’s 557 enrollees, split fairly evenly across the two cohorts. Cutting the PFS benefit this way yields a 53% reduction in risk of progression or death in patients with CNS involvement, versus a much smaller 25% reduction (with a confidence interval upper bound over 1.00) in those without.
Astra told ApexOnco that, while it viewed patients with CNS metastases as those with the greatest unmet need and potential to benefit from a chemo combo, "the Flaura2 regimen should be considered as a potential treatment option for patients who do not have CNS mets".
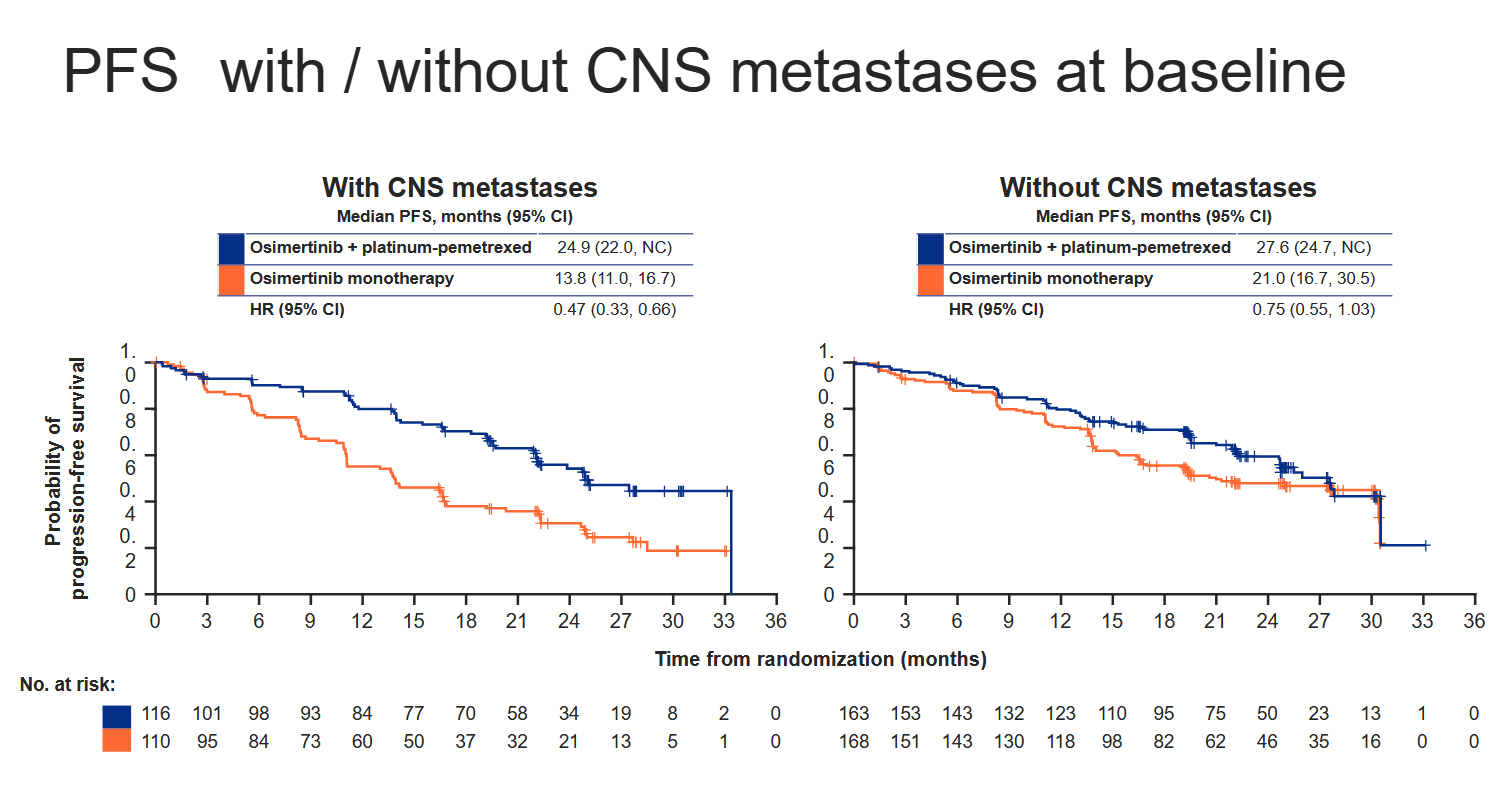
Looking at CNS involvement was a prespecified subgroup analysis in Flaura2, Astra said. Another important baseline characteristic in the trial is WHO performance status, with less sick patients apparently deriving a lesser benefit, according to Flaura2’s spider plot.
It must also be stressed that nothing has been presented regarding OS benefit across subgroups. Indeed, for the entire trial this analysis is only 27% mature, and the early survival curves cross over, without a hint of statistical significance (HR=0.90, p=0.5).
Astra said that, given high levels of censoring, no conclusions could be drawn about OS, a secondary Flaura2 endpoint, though it called the current trend positive. PFS results as they stand "support a regulatory submission", it told ApexOnco.
Still, while any doubts persist, J&J’s Rybrevant plus lazertinib clearly cannot be written off. Mariposa, having passed interim analysis, is expected to read out finally by the end of the year, so all J&J needs now is for it to work.
This story has been updated to add discussant comments.
2038













