
ASCO 2023 – Bristol’s Commands data look good for Geron
The limited activity of Reblozyl in myelodysplastic syndromes patients without ringed sideroblasts could help Geron.
The limited activity of Reblozyl in myelodysplastic syndromes patients without ringed sideroblasts could help Geron.

Data being presented at ASCO from the front-line Commands trial have confirmed the limited activity of Bristol Myers Squibb’s Reblozyl in patients without ringed sideroblasts. The finding could favour Geron’s imetelstat, until now seen as the underdog in this growing battle.
Though a pre-ASCO press briefing talked up the data, the results seem pretty unambiguous, with no benefit at all in ringed sideroblast-negative subjects on Commands’ primary endpoint. These patients make up around two thirds of this MDS population, and crucially Geron’s Imerge trial suggested that imetelstat was active in them.
That said, the rival studies are not identical in design. Commands is a first-line MDS trial of Reblozyl against erythropoiesis-stimulating agents (ESAs), while Imerge tested imetelstat in subjects who had progressed on, or were ineligible, for ESAs.
In the US Reblozyl is approved in low-risk MDS for use after ESAs, though only in ringed sideroblast (RS) positive patients; nevertheless, the drug is apparently prescribed off label in some RS-negative MDS patients too.
Replacing ESAs?
At the ASCO press briefing Dr Guillermo Garcia-Manero, from the MD Anderson Cancer Center, said Reblozyl could replace ESAs as a new front-line option thanks to Commands, which he called a “paradigm shift in the treatment of low-risk MDS-associated anaemia”.
His enthusiasm was driven by the fact that Reblozyl was nearly twice as likely to result in transfusion independence with haemoglobin increase than epoetin alfa. 58.5% of Reblozyl recipients achieved this at 12 weeks, Commands’ primary endpoint, versus 31.2% of patients given epoetin alfa (p<0.0001).
Nevertheless, as is often the case in heterogeneous patient populations, this all-comers effect was clearly driven by RS-positive patients. RS-negative subjects derived no added benefit, with 12-week transfusion independence of 41.0% for Reblozyl versus 46.3% for epoetin alfa.
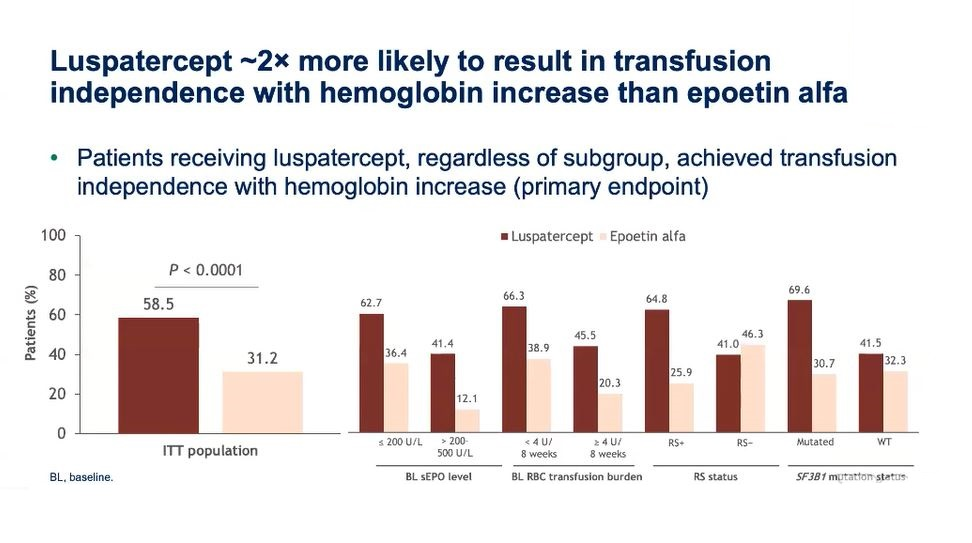
In spite of this unavoidable fact, Garcia-Manero said Reblozyl should be used irrespective of RS status. He told the briefing that Commands did not reflect the real-world population because over 60% of enrolled patients were RS-positive, and that the trial was “not powered to see a big difference ... in the RS-negative context”.
He also highlighted duration of transfusion independence, a secondary Commands endpoint that he suggested was as important as response itself. Here there was a strong numerical benefit favouring Reblozyl versus epoetin alfa among all-comer, RS-positive and RS-negative patients.
Either way, Commands is an important trial. It will likely result in Reblozyl getting a front-line label in low-risk MDS, though how broad this is has yet to be determined. Depending on the extent to which the Bristol drug replaces front-line ESAs it will change the second-line treatment landscape.
But for Geron the key is that, as long as Reblozyl is not indicated for RS-negative MDS, the second-line imetelstat opportunity should remain intact, a scenario that would hit Reblozyl’s potential peak sales. And a recent KOL survey cited by B Riley analysts suggested that prescribers would use the Geron drug in 55% of post-Reblozyl MDS, even though it has yet to be tested in these patients.
Garcia-Manero said Reblozyl’s response duration, relative safety and ease of SC administration every three weeks would likely make the drug “the standard of care for the majority of [low-risk] patients, regardless of whether they are RS-positive or negative”, but it remains to be seen whether the FDA shares such enthusiasm.
229













