ASCO 2023 – Servier’s Indigo win comes with liver tox
Toxicity casts a shadow over vorasidenib’s prospects as possibly the first targeted therapy for low-grade glioma.
Toxicity casts a shadow over vorasidenib’s prospects as possibly the first targeted therapy for low-grade glioma.

Servier spent $1.8bn buying Agios Pharmaceuticals’ cancer portfolio, and the lead asset in that move, vorasidenib, just scored in its phase 3 Indigo trial. This could go some way towards justifying the acquisition price – at least in terms of the IDH1/2 inhibitor’s efficacy, which backs its potential as the first targeted treatment for low-grade glioma.
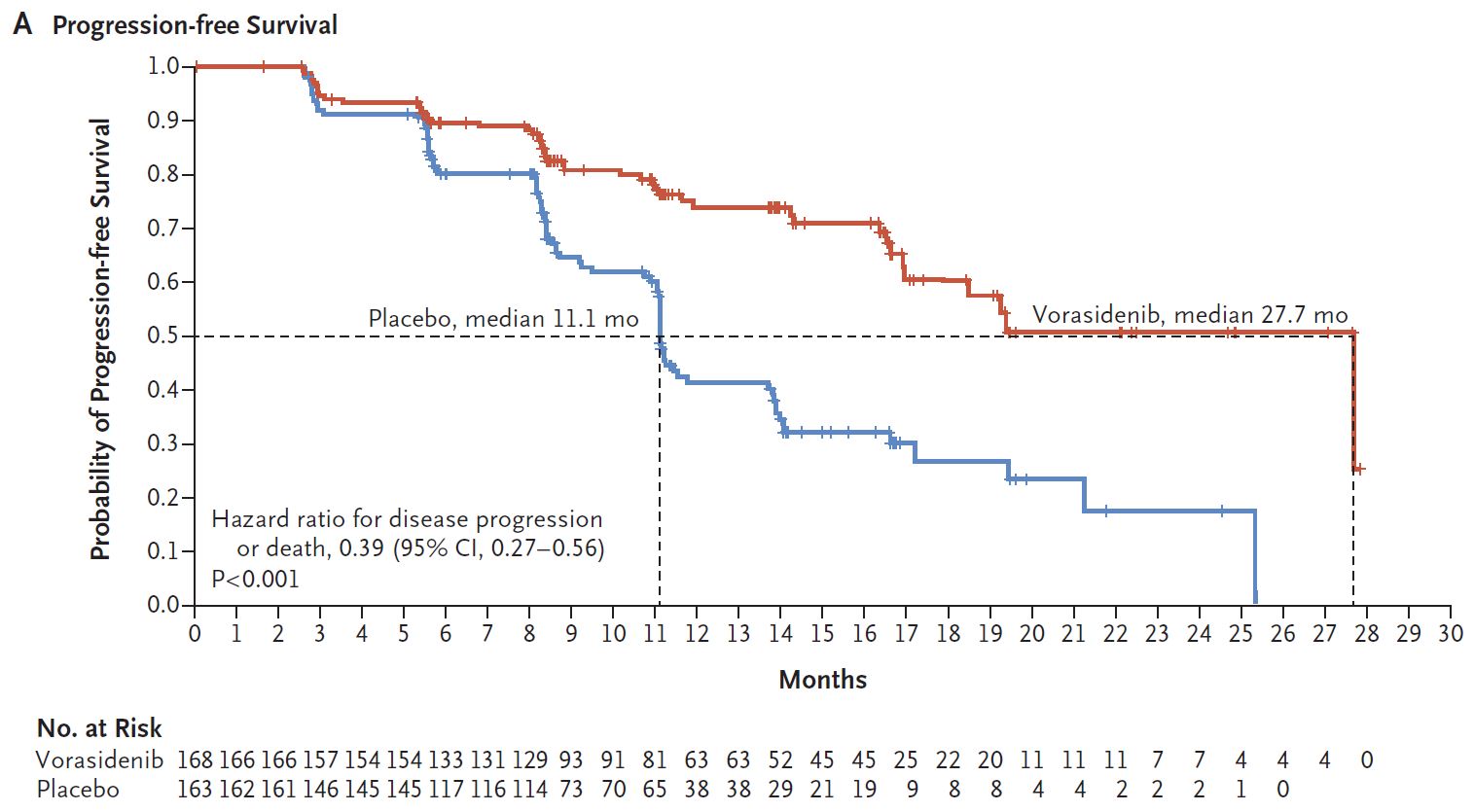
That efficacy looks impressive, with patients’ risk of progression or death being cut by 61% versus placebo, ASCO data suggest. However, this has come with significant levels of liver toxicity, the most concerning of which comprises two cases of liver enzyme elevation that were severe enough to trigger Hy’s law, a rule of thumb suggesting patients are at risk of fatal drug-induced liver injury.
Presenting the data, doctors stressed that the liver enzyme elevations were transient. The Indigo trial’s co-author, Dr Patrick Wen of the Dana-Farber Cancer Institute, called the hepatotoxicity very manageable and reversible, stating: “The [vorasidenib] dose can be reduced if [toxicity] is severe, and relatively few patients stop the drug because of the hepatotoxicity.”
Meanwhile, Susan Pandya, Servier’s oncology head, confirmed that the liver enzyme increases were “reversible and [could] be monitored through routine blood work as part of the clinical management of the patient”, and said the firm did “not foresee this as having an impact on the success of vorasidenib”.
Biomarker-positive
The setting of Indigo was patients with grade 2 glioma that was positive for IDH1/2, a mutation that occurs in most of these low-grade cancers, and had been surgically removed within the previous one to five years. The trial tested vorasidenib’s ability to delay the need for radiation or chemotherapy versus watchful waiting, the current standard.
Vorasidenib showed a median PFS of 27.7 months versus with 11.1 months for placebo on this primary endpoint, producing an impressive and highly statistically significant hazard ratio of 0.39, today’s ASCO late-breaker revealed.
On the other hand, 39% of patients given vorasidenib experienced elevations in alanine aminotransferase, and in 10% of patients receiving the IDH1/2 inhibitor this adverse event was seen at grade 3 or above, an accompanying NEJM paper revealed. After the presentation Servier disclosed that two of the elevation cases were severe enough to trigger Hy’s law.
While Servier expects approvals in the second half of 2024, whether these happen this will depend on how the FDA views liver toxicity in this cancer setting.
Agios itself stands to benefit in the event of a regulatory green light, being eligible for a $200m milestone payment on vorasidenib’s US approval, plus 15% royalties. Servier also now plans to expand vorasidenib development to other forms of glioma.
As well as vorasidenib, Servier’s Agios cancer pipeline acquisition brought the IDH1 inhibitor Tibsovo, approved for IDH1-positive acute myelogenous leukaemia and cholangiocarcinoma. The competitor pipeline includes several inhibitors of IDH1 and IDH2, but hitting both mutations seems to be essential for activity in glioma; Hutchmed’s phase 1 asset HMPL-306 apparently has such dual activity too, but there are not many others.
306













