
ASCO-GU – Pfizer looks for bigger bladder cancer invasion
Padcev still looks good in first-line bladder, but Pfizer hopes to make a mark in muscle-invasive disease.
Padcev still looks good in first-line bladder, but Pfizer hopes to make a mark in muscle-invasive disease.

Since bagging FDA approval in first-line bladder cancer in late 2023 based on the EV-302 trial, Pfizer and Astellas’s Nectin-4-targeting ADC Padcev has become a standard of care, and the companies will hope to cement that position with longer-term data from the same trial being presented at the ASCO Genitourinary Cancers Symposium this week.
After an additional year of follow-up, the study found a 49% reduction in the risk of death with Padcev plus Keytruda, versus chemo – a slight slippage from the 53% reduction previously reported. However, more uses could be beckoning, with Pfizer recently highlighting pivotal readouts that, it says, could triple the US patient population addressable by Padcev.
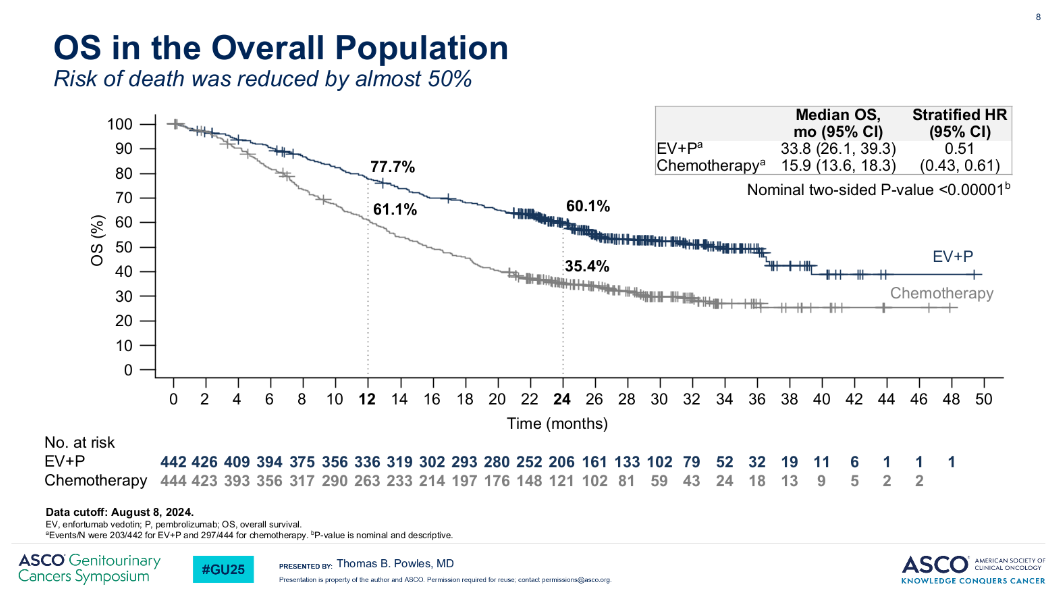
During its fourth-quarter earnings call last week the company flagged two phase trials in the pre-metastatic muscle-invasive bladder cancer (MIBC) setting that could, if all goes well, yield interim data this year: EV-303, in cisplatin-ineligible, and EV-304, in cisplatin-eligible disease.
Both combine perioperative Padcev plus Keytruda plus cystectomy – bladder removal surgery that’s the current gold standard in this disease. The primary endpoint of both studies is event-free survival.
Keytruda has already shown a benefit in muscle-invasive bladder cancer, in the NCI-sponsored Keynote-123 trial. Last year’s ASCO-GU meeting heard that adjuvant Keytruda reduced risk of disease recurrence or death by 31% versus observation after surgery. However, there has been no news of a filing as yet, and when asked about the regulatory strategy for Keynote-123 a Merck spokesperson declined to comment.
Imfinzi combo?
As well as Keytruda, Padcev could end up being combined with AstraZeneca’s checkpoint inhibitor Imfinzi in MIBC, with Astra’s Volga study also set to read out in the second half of this year.
Meanwhile, Johnson & Johnson’s gemcitabine-eluting project TAR-200 yielded data from the phase 2 Sunrise-4 trial at last year’s ESMO meeting, in MIBC patients undergoing cystectomy. However, the phase 3 Sunrise-2 trial, in MIBC patients ineligible for or refusing to undergo cystectomy, was discontinued for futility in October.
Still, the bigger pre-metastatic use is non-muscle invasive bladder cancer (NMIBC), which is becoming an increasingly crowded space, with approvals for Keytruda, Ferring’s Adstiladrin and ImmunityBio’s Anktiva.
CG Oncology's oncoltyic virus candidate cretostimogene grenadenorepvec could soon be heading to regulators, while Astra and J&J also have phase 3 trials ongoing. Pfizer looks behind here, with the phase 1 EV-104 study, testing intravesical Padcev, ongoing.
Notable Padcev trials
| Trial | Setting | Regimen | Note |
|---|---|---|---|
| EV-302 | 1st-line locally advanced/metastatic urothelial cancer | + Keytruda, vs chemo | Data at ESMO 2023: 53% reduction in risk of death; FDA approved Dec 2023; data at ASCO GU 2025: 49% reduction in risk of death |
| EV-303 | Periadjuvant MIBC (cisplatin-ineligible) | + Keytruda + surgery, vs Keytruda + surgery or surgery alone | Interim data due 2025 |
| EV-304 | Periadjuvant MIBC (cisplatin-eligible) | + Keytruda + surgery, vs neoadjuvant chemo + surgery | Interim data due 2025 |
| Volga | Periadjuvant MIBC (cisplatin-ineligible) | + Imfinzi + surgery +/- Imjudo, vs surgery | Data due H2 2025 |
MIBC=muscle-invasive bladder cancer. Source: OncologyPipeline, clinicaltrials.gov & Pfizer Q4 2024 presentation.
1582













